
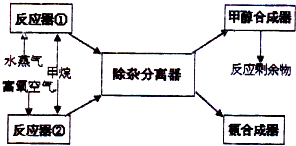
 ij�������Լ���Ϊ��Ҫԭ������ȡ�ϳɼ״����ϳɰ���ԭ�ϣ�����Ҫ��ӦΪ��
ij�������Լ���Ϊ��Ҫԭ������ȡ�ϳɼ״����ϳɰ���ԭ�ϣ�����Ҫ��ӦΪ��| 2 |
| 3 |
| n(H2) |
| n(N2) |
| m |
| M |
| 672��103L |
| 22.4L/mol |
| 3��672��103 |
| 22.4L/mol |
| 2 |
| 3 |
| 2 |
| 3 |
| 2 |
| 3 |
| 2 |
| 3 |
| 4 |
| 3 |
| 1 |
| 3 |
| 4 |
| 3 |
| 1 |
| 3 |
| 1 |
| 3 |
| 1 |
| 3 |
| 1 |
| 3 |
| 1 |
| 3 |
| 1 |
| 1+1 |
| 5 |
| 12 |
| 5 |
| 12 |
| 5 |
| 12 |
| 5 |
| 12 |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ij��ѧʵ��С����г�������һƿijƷ��ʳ�ð״ף���Ҫ�Ǵ����ˮ��Һ������ʵ���ұ�NaOH��Һ������еζ��Բⶨ����ȷŨ�ȣ���ȫ��Ӧʱ������ҺpH����Ϊ9���±���4�ֳ���ָʾ���ı�ɫ��Χ��
ij��ѧʵ��С����г�������һƿijƷ��ʳ�ð״ף���Ҫ�Ǵ����ˮ��Һ������ʵ���ұ�NaOH��Һ������еζ��Բⶨ����ȷŨ�ȣ���ȫ��Ӧʱ������ҺpH����Ϊ9���±���4�ֳ���ָʾ���ı�ɫ��Χ��| ָʾ�� | ʯ�� | ���� | ���� | ��̪ |
| ��ɫ��Χ��pH�� | 5.0��8.0 | 3.1��4.4 | 4.4��6.2 | 8.2��10.0 |
| ʵ����� | ��һ�� | �ڶ��� | ������ |
| ����NaOH��Һ���/mL | 26.02 | 25.35 | 25.30 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��0.1mol?L-1��NaOH��Һ��0.1mol?L-1��H2SO3��Һ����Ϻ�����ԣ���c��H2SO3����c��SO32-�� |
| B��0.1mol?L-1��NaOH��Һ��0.1mol?L-1�Ĵ�����Һ��c��Na+����c��CH3COO-�� |
| C��pH=3�������pH=11�İ�ˮ��c��OH-����c��H+�� |
| D��0.1mol?L-1��NH4Cl��Һ��0.1mol?L-1�İ�ˮ����Ϻ�ʼ��ԣ���c��NH4+����c��Cl-�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ����X2��Y�ͻ�����Z�����Ԫ�ؾ�Ϊ���ڱ���ǰ20��Ԫ�أ������ͼ�ֱ���X2��Y ��Z���й���Ϣ�Լ�����֮���ת����ϵ ����������������ȥ������ش��������⣺
����X2��Y�ͻ�����Z�����Ԫ�ؾ�Ϊ���ڱ���ǰ20��Ԫ�أ������ͼ�ֱ���X2��Y ��Z���й���Ϣ�Լ�����֮���ת����ϵ ����������������ȥ������ش��������⣺| Ԫ��X | ���γ�-1��18���ӵĵ������� |
| Ԫ��Y | ��3���ڵڢ�A�� |
| ������Z | ������������3�����Ӳ� |
| ������0.1mol?L-1Z��ˮ��ҺpH=13 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A����������ϡ���ᷴӦ��OH-+H+=H2O |
| B��С�մ������ᷴӦ��CO32-+2H+=CO2��+H2O |
| C��Fe�����ᷴӦ��2Fe+6H+=2Fe3++3H2�� |
| D������CO2ͨ��NaOH��Һ��CO2+2OH-=CO32-+H2O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com