
����Ŀ���밴Ҫ����գ�
��1����![]() ��������___��
��������___��
��![]() ��������___��
��������___��
�۱�����Ũ��ˮ��Ӧ�Ļ�ѧ����ʽ��___��
�ܼ�ȩ������������ͭ����Һ��Ӧ�Ļ�ѧ����ʽ��___��
��ij�л�������A�ķ���ʽΪC5H11Br�����ӽṹ����������CH3������![]() ��һ����Br����A�Ľṹ��ʽΪ___��
��һ����Br����A�Ľṹ��ʽΪ___��
��2��ij�л���A����Է�������Ϊ74���Һ������ͼ��ͼ����A�Ľṹ��ʽΪ__��
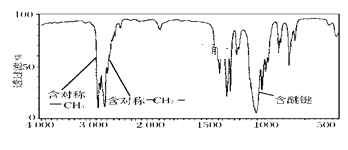
��3��1���ij����������ȫȼ�����ɵ�CO2�����ɵ�ˮ������1�������ͬ��ͬѹ�²ⶨ����0.1mol������ȫȼ�յIJ��ﱻ��ʯ�����գ���ʯ������39g�������ķ���ʽΪ__����������һ�ȴ�����3�֣�д���������п��ܵĽṹ��ʽ__��
���𰸡�3-��-2-���� 2-��-2��4-����ϩ ![]() +3Br2
+3Br2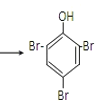 ��+3HBr HCHO+4Cu(OH)2+2NaOH
��+3HBr HCHO+4Cu(OH)2+2NaOH![]() 2Cu2O��+Na2CO3+6H2O CH3CHBrCH(CH3)2 CH3CH2OCH2CH3 C6H14 CH3(CH2)4CH3��(CH3)3CCH2CH3
2Cu2O��+Na2CO3+6H2O CH3CHBrCH(CH3)2 CH3CH2OCH2CH3 C6H14 CH3(CH2)4CH3��(CH3)3CCH2CH3
��������
��1���ٸ��ݴ������������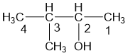 ������Ϊ��3-��-2-������
��������3-��-2-������
�ڸ��ݶ�ϩ������������![]() ������Ϊ��2-��-2��4-����ϩ��
������Ϊ��2-��-2��4-����ϩ��
�۱�����Ũ��ˮ����ȡ����Ӧ��Br2ȡ�����ӷ����ϵ�������λ��һ����λ����Ӧ�Ļ�ѧ����ʽΪ��![]() +3Br2
+3Br2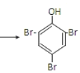 ��+3HBr��
��+3HBr��
�ܼ�ȩ������������ͭ����Һ����������Ӧ������ש��ɫ��Cu2O���������ڼ�ȩ��2��ȩ����������4������Cu(OH)2��Ӧ����ѧ����ʽΪ��HCHO+4Cu(OH)2+2NaOH![]() 2Cu2O��+Na2CO3+6H2O��
2Cu2O��+Na2CO3+6H2O��
�ݸ������⣬ij�л�������A�ķ���ʽΪC5H11Br�����ӽṹ����������CH3������![]() ��һ����Br��A�Ľṹ��ʽΪ��CH3CHBrCH(CH3)2��
��һ����Br��A�Ľṹ��ʽΪ��CH3CHBrCH(CH3)2��
��2�����л�������ԭ������Ϊ74���������ͼ��ʾ���жԳƵļ����ԳƵ��Ǽ����Ѽ�������л���Ľṹ��ʽΪCH3CH2OCH2CH3��
��3�������ķ���ʽΪCxHy��ȼ�շ���ʽΪ��
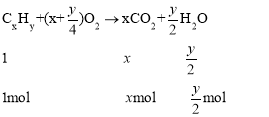
���![]() �����Ը�������ʽΪC6H14�����ڸ�����һ�ȴ��������֣��������ֲ�ͬ������H������ṹ��ʽΪCH3(CH2)4CH3��(CH3)3CCH2CH3��
�����Ը�������ʽΪC6H14�����ڸ�����һ�ȴ��������֣��������ֲ�ͬ������H������ṹ��ʽΪCH3(CH2)4CH3��(CH3)3CCH2CH3��


 �Ͻ�ƽ���Ȿϵ�д�
�Ͻ�ƽ���Ȿϵ�д� ����ѧ��Ӧ�����ϵ�д�
����ѧ��Ӧ�����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
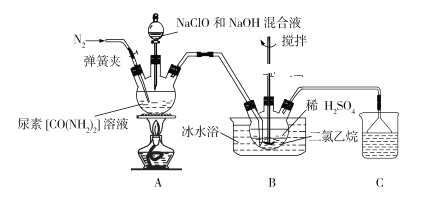
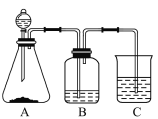
����Ŀ��ij���п���С��ģ�ҵ���ط�������(N2H4)�����巽�����Ƚ����ء��������ƺ�����������Һ��ϣ�Ȼ���ڴ������ڵ������·�Ӧ�Ƶ��£����õ�������������е��£��������������Ʊ������¡�����ʵ��ԭ��ͼ���£�
��֪�����¼�������ˮ�����ڱ�¶�ڿ����л��ʱ���ܸ������ûᱬը�ֽ⡣
��������(N2H4��H2SO4)��������NH4HSO4�����࣬��ɫ���壬������ˮ����������ˮ���������Ҵ��Ͷ���������л��ܼ���
���ܶȣ���������>����������>��ϡ����
(1)ʢװ������Һ��װ������Ϊ_____________________�������µ�ˮ��Һ�к��ж��������ӣ�����������N2H5+�ĵ���ʽΪ________________________��
(2)��ӦǰӦ��ͨ��һ��ʱ�䵪������Ŀ��Ϊ________________________________��
(3)װ��A�з�����Ӧ�Ʊ��µ����ӷ���ʽΪ_______________________________��
(4)װ��B�ж������������Ϊ__________________________________________��ʹ�ñ�ˮԡ������__________________________________________________��
(5)װ��B��Ӧ��ȫ���辭�����ˡ�ϴ�ӡ�����Ȳ����õ������£�ϴ�ӹ��������ѡ����������ϴ�Ӽ�________________(����ĸ)��
A����ˮ B����ˮ C����ˮ�Ҵ� D������ʳ��ˮ
(6)��ʵ�������õ�NaClO��NaOH���Һ�������ʵ�Ũ�Ⱦ�Ϊ0.1mol��L1�������Һ������Ũ�ȵĴ�С˳��Ϊ______________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����淴ӦH2(g)+I2(g)2HI(g)��һ���������·�Ӧ������и���ֵ�Ũ��(mol��L-1)��ʱ��t(min)�ı仯������ͼ��ʾ������ͼʾ���ش�
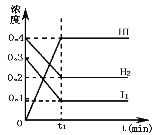
(1)ʱ��t1��������_________
(2)��ʱ��0��t1�ļ���v(H2) =________________
(3)��ƽ��״̬�£�H2��ת����Ϊ_________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��SO2�����ڷ���������������Ҳ��һ����Ҫ���䶳���ʡ�ʵ���ҿ�����ͼ��ʾװ���Ʊ�SO2�����ô���SO2�������ʵ�顣
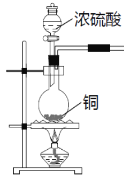
(1)���������Ʊ���SO2�У��������������СҺ�ζ��ʰ���״����ȥ���������Ʊ�װ�ú�������ͼ��ʾװ�ã���װ���е��Լ���__________�������_______��(����a������b��)����
(2)����SO2���õ��Լ���__________________��������SO2��_________�ԡ�
(3)��SO2ͨ��0.1mol/L Ba(NO3)2��Һ�У��õ���ɫ�������ó����Ļ�ѧʽΪ___________��
�ֱ�����к�δ��й�������ˮ����Ba(NO3)2��BaCl2��Һ����������ʵ�飺
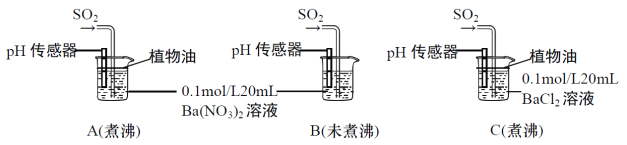
(4)ʵ��A��C�У��������ˮ��ʹ��ֲ���͵�Ŀ����_________________________________��
(5)ʵ��C�У�û�й۲쵽��ɫ��������pH��������ʾ��Һ�����ԣ�ԭ����__________________��(�÷���ʽ��ʾ)
(6)ʵ��B�г��ְ�ɫ������ʵ��A��ܶࡣ�ɴ˵ó��Ľ�����___________________________����ʵ��A��B��ͨ��������SO2����ҺpH��A_________B(����>������<������=��)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
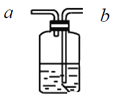
����Ŀ��Ϊ��ȷ�����ᡢ̼����������ǿ���������������ͼ��ʾװ�ã�һ��ʵ��ﵽĿ��(������ѡ������������Һ)��
(1)��ƿ��װ��ij�ֿ��������ι���(����3��������������һ��)���˹���Ϊ________����Һ©������ʢ�Լ���___��
(2)װ��B����ʢ�Լ���������________���Լ���������__________��
(3)װ��C�г��ֵ�������__________________��
(4)��ʵ���֪����������Դ�С˳��Ϊ________(�û�ѧʽ��ʾ)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��![]() ��һ���л�ϩ�ѣ���������Aͨ������·���Ƶã�
��һ���л�ϩ�ѣ���������Aͨ������·���Ƶã�
![]()
������˵����ȷ���ǣ� ��
A.A��������2-��ϩ
B.B�����в��������ͼ�
C.C��һ�ֶ�Ԫ����������ˮ
D.3�ķ�Ӧ����Ϊ��ȥ��Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ɫ�Ļ������ף����ܺ�NO��CO2��NO2��N2�еļ��֣���һ�����ļ����徭����ͼʵ��Ĵ���������õ�������Һ��������������ʣ�࣬�����������Ϊ�� ��
A.NO2��N2B.NO��CO2
C.NO2��CO2D.NO��CO2��N2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����һ�������£���6�����NO2(������N2O4)��һ�������NO�����Թ��У������Թܵ�����ˮ�У���ͨ��4.5�����O2��ַ�Ӧ��ʣ��1.5������壬��ԭNO�������Ϊ(����)
��3�������4�������5�������5.5�������2���
A.��B.��C.�ܻ��D.�ڻ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ѧ��ѡ��2����ѧ�뼼������ú����ȼú�糧�ų�����Ҫ�������ҹ���糧��ú�ҵ���Ҫ���������Ϊ��SiO2��Al2O3��CaO�ȡ�һ�����÷�ú����ȡ�������Ĺ����������£�

��1����ú����ĥ��Ŀ���� ��
��2����1�ι�����������Ҫ�ɷ��� �� ���ѧʽ, ��ͬ��,��3�ι���ʱ�������ijɷֵ��� ��
��3����104���������ȡʱ�����Ľ�ȡ����ʱ��Ĺ�ϵ����ͼ1�����˵Ľ�ȡʱ��Ϊ h�����Ľ�ȡ���롰���ܼ�/��ú�ҡ��Ĺ�ϵ��ͼ2��ʾ���ӽ�ȡ�ʽǶȿ��ǣ��������ܼ�NH4F��KF����NH4F��KF�Ļ��������ܼ�/��ú����ͬʱ����ȡ����ߵ��� ���ѧʽ�����ú����Ļ��������������ܼ�ȱ���� ����һ������

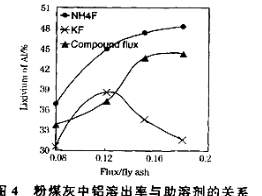
��4��������ѭ��ʹ�õ������� �� ���ѧʽ����
��5���������ܽ����������壬�ܹ�������ԭ���� ��
��6���÷�ú����ȡ�������������Ҫ������ ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com