
��Ȥ�Ļ�ѧʵ���ܼ���ѧ��ѧϰ��ѧ����Ȥ�����ѧϰ�����ԣ�ͬʱҲ��Ծ�������ա�����ѧ��ѧ�̲��У������ھ�������Ȥ��ʵ�飬���磺
��.[������Ӿ]ˮ�����ݣ��������ǹ��ϵij�ʶ������û�п�������������ˮ����������ȥ��Ȼ����ij��ѧ��ȤС���ͬѧ������һ����������Ӿ��Сħ��������һ��100�������ձ�����װ������ˮ��Ȼ�������ӽ�һ�鶹����Ľ����أ�����ط����ձ����ʱ���ձ����ˮ�����������ɸ��������������ˮ���Ϲ�����ȥ��ͬʱ�������͵�����������ʮ��׳�ۡ�
��.[����ƿ�ӡ�ʵ��]��250 mLƽ����ƿ�����μ���2 g�������ơ�100 mL����ˮ��2 g������(��ĩ)����ʹ���ܽ⡣����4��6��0.2%���Ǽ�����Һ������ƿ��(��ͼ)������ƿ����Һ����ɫ�����ã�Լ3���Ӻ���ɫ��ʧ����Ϊ��ɫ���ٴ�����ɫ��Һ�ֳ���ɫ���ɷ�����Ρ���֪���Ǽ������ױ���ԭ����������ʵ�飬�ش��������⣺
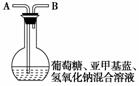
(1)ʵ�����н�����Ӧ������______________________��ԭ����________________________________����ѧ��ȤС���ͬѧȡ��һС�������صIJ�����________________________________________________________________________
________________________________________________________________________��
(2)д�����н����ط�����Ӧ�����ӷ���ʽ�� __________________________________
________________________________________________________________________��
(3)Ϊ̽������ƿ�ӡ�ʵ����ʲôԭ������ģ�ijͬѧ��������ʵ�飺
A������ƿ��ȡ����Һע��һ֧�Թܣ�������Ƥ�����Թ��е���Һ����ɫ��Ϊ��ɫ�������Թ���Һ���ܱ�����
B����ԭ��ƿ�о�A�������������Һ�����������ˡ�����Ƭ�̺���ɫ����ʧ���ٴι��������������������
C��Ϊ�˽�һ��Ū���Ǻ��ֳɷ�����ģ����ּ���̽������A����ͨ������N2��CO2��������ʹ��ƿ�е���ɫ��Һ���������Ƶõ�������A��ͨ�룬����������ɫ��Һ������Ϊ��ɫ��
����Ϊֹ������ƿ�ӡ�ʵ��ԭ�����˿�ѧ���ۣ������ܽᡰ��ƿ�ӡ�ʵ��ı�ɫԭ����________________________________________________________________________
________________________________________________________________________��
����������ʵ�����Ҫ��Ͽα�ѧϰ�Ľ�����֪ʶ����һ����չ�����ѻش�����⡣ʵ���Ҫ���̽�������н������С��������ޡ������������һ���뵽��ԭ������֮��Ĺ�ϵ��
�𰸣�(1)ú�ͻ�ʯ��������غܻ��ã����������е�������ˮ���������ʷ�Ӧ�������Ӵ��Լ�ƿ��ȡ��һ������أ�������ֽ�ϰ�ú�����ɣ���С����ȥһС�������أ�ʣ��ķŻ��Լ�ƿ��
(2)2K��2H2O===2K����2OH����H2��
(3)�����Ǽ����������ǻ�ԭ����ԭ�����ɫ���ʣ��ֱ������������������ֳ�Ϊ�Ǽ�����������ɫ


 ��У��������ĩ��̾�ϵ�д�
��У��������ĩ��̾�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����ڼ�Դ����ʻ�ʩ����S-�տ����Ƽ����Ա�֤�ʻ�ʢ����S-�տ��صķ��ӽṹ��ͼ��˵����ȷ����
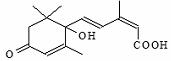 A������ʽC15H21O4
A������ʽC15H21O4
B�����ܷ����Ӿ۷�Ӧ�����ܷ������۷�Ӧ
C�������������ֹ�����
D������ʹ�Ȼ�����Һ������ɫ��Ӧ������ʹ���Ը��������Һ��ɫ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����˵���У�����ȷ����
A��HF�ȶ��Ա�HClǿ B��Mgʧ����������Ca��
C��NaCl��NH4Cl���еĻ�ѧ��������ͬ D�������ʵ�����C2H6��H2O2����������ͬ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ʾ���ڴ�����ȼ��ʵ��ʱ������˿�Ƴ�����״������ҪĿ����(����)
A�������˿������
B����߷�Ӧ�¶�
C������Ӧ�ĽӴ���
D��ʹƿ�������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��������ʵ�飺����ƿa�з���1 g�̶������̼��ƣ�����ƿb�м���1 g��ĩ״��̼��ƣ��ֱ����50 mL 1 mol·L��1�����ᡣ��ͼ��ʾ(x��ʾʱ�䣬y��ʾ�������������)������ȷ��ʾʵ��������(����)

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���д�ʩ����ʵ��������������ԭ�����͵��� ( )
A�����Ƶ���ˮ�ڹ�������ɫ��dz
B��Fe(SCN)3��Һ�м���6mol/L NaOH��Һ����ɫ��dz
C���ںϳɰ��ķ�Ӧ�У����»��ѹ�����ڰ��ĺϳ�
D��H2��I2��HIƽ��������ѹ����ɫ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����й�ʵ���˵������ȷ���� ( )
A���ڲⶨ�кͷ�Ӧ�ķ�Ӧ��ʵ���У�Ҫ��ȡ����¶�
B���к͵ζ�ʵ���У�ϴ�������ƿ����Ҫ����
C����CH3COONa��Һ�е���ʯ����Һ����Һ����
D�����ɫZnS�����ϵμ�CuSO4��Һ��������Ϊ��ɫ��˵��Ksp(ZnS)��Ksp(CuS)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ͼ��ʾװ�ý���ʵ�飬���ش��������⣺
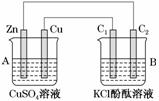 (1)�ж�װ�õ����ƣ�A��Ϊ________��B��Ϊ________________��
(1)�ж�װ�õ����ƣ�A��Ϊ________��B��Ϊ________________��
(2)п��Ϊ________�����缫��ӦʽΪ__________________��
ͭ��Ϊ________�����缫��ӦʽΪ________________________________________
________________________________________________________________________��
ʯī��C1Ϊ____�����缫��ӦʽΪ______________________________________
________________________________________________________________________��
ʯī��C2����������ʵ������Ϊ________________________________________
________________________________________________________________________��
(3)��C2������224 mL����(��״��)ʱ��п������________(����ӡ����١�)________g��CuSO4��Һ������________(����ӡ����١�)________g��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ijѧ����������ƽ����ʱ����ҩƷ�������λ�÷ŵߵ��ˣ���ƽ��ʱ���Ƶ�ҩƷ�����Ķ���Ϊ9.5g��1g���������룩����ҩƷ��ʵ������Ϊ����
| A�� | 9g | B�� | 10g | C�� | 8.5g | D�� | 8g |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com