�⣺��1��2 I
2��s��+5O
2��g��=2 I
2O
5��s������H=-75.56kJ?mol
-1�٣�
2CO��g��+O
2��g��=2 CO
2��g������H=-566.0kJ?mol
-1�ڣ�
������ʽ�ڡ�
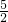

��5CO��g��+I
2O
5��s��=5 CO
2��g��+I
2��s������H=��-566.0kJ?mol
-1����
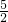
-��-75.56kJ?mol
-1����

=-1377.22kJ/mol��
�������Ȼ�ѧ��Ӧ����ʽΪ��5CO��g��+I
2O
5��s��=5 CO
2��g��+I
2��s������H=-1377.22kJ/mol��
�ʴ�Ϊ��5CO��g��+I
2O
5��s��=5 CO
2��g��+I
2��s������H=-1377.22kJ/mol��
��2��ԭ��طŵ�ʱ���������Һ�м������������ƶ��������������õ��Ӻ�ˮ��Ӧ�������������ӣ����Ե缫��ӦʽΪ��O
2+2H
2O+4e
-=4OH
-��
�ʴ�Ϊ������O
2+2H
2O+4e
-=4OH
-��
��3���ٸø��Ϸʺ���N��PԪ�أ�Ϊ����炙�����һ��炙��������泥��ʴ�Ϊ����NH
4��
3PO
4����NH
4��
2HPO
4��NH
4H
2PO
4��
��笠����ӵ�ˮ��ƽ�ⳣ��K=
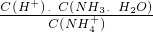
=
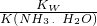
��
�����������ˮ��ƽ�ⳣ��K��=

=
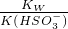
��
һˮ�ϰ��ĵ���ƽ�ⳣ������������������ӵĵ��� ƽ�ⳣ��������笠����ӵ�ˮ��ƽ�ⳣ��С�������������ˮ��ƽ�ⳣ�����������������ˮ��̶ȴ���笠�����ˮ��̶ȣ�������Һ�ʼ��ԣ�
�ʴ�Ϊ���
����Һ�ʼ��ԣ�Ҫʹ������Һ�����ԣ����Լ����������壬���Լ������������Һ�����ԣ���Һ��������Ũ�ȵ�������������Ũ�ȣ���Һ�л�����������������ӣ�������ɣ����ݵ���غ�֪��笠�����Ũ�ȴ��������������Ũ�ȵ�2����
�ʴ�Ϊ��SO
2������
�����������£��������Ӻ���������ӷ�Ӧ���������ӡ�һ��������ˮ�����ӷ�Ӧ����ʽΪ��3Fe
2++NO
3-+4H
+=3Fe
3++NO��+2H
2O��
�ʴ�Ϊ��3Fe
2++NO
3-+4H
+=3Fe
3++NO��+2H
2O��
��������1�����ݸ�˹���ɽ�����д��
��2��ԭ��ص������Һ���������������ƶ���
����������ԭ��Ӧ��������ͨ�룬���������������������ŵ��������������ӣ�
��3���ٸø��Ϸʺ���N��PԪ�أ�Ϊ����炙�����һ��炙��������泥�
�ڰ�ˮ��SO
2ǡ����ȫ��Ӧ�������Σ���Ӧ����������泥����ݵ���ƽ�ⳣ���ж����ӵ�ˮ��ƽ�ⳣ��������ˮ��̶���Դ�Сȷ����Һ������ԣ�
����Һ�ʼ��ԣ���Һ��Ӧ�ü�����������ʹ���Ϊ���ԣ����ݵ���غ��жϣ�
�����������£��������Ӻ���������ӷ�Ӧ���������ӡ�һ��������ˮ��
�����������漰��˹���ɡ�ԭ���ԭ����������ԭ��Ӧ������ˮ���֪ʶ�㣬�ѵ����ж�����������Һ������ԣ�������ˮ��ƽ�ⳣ���жϣ���ν�ˮ��ƽ�ⳣ����������ʵĵ���ƽ�ⳣ����ϵ�ǽ⣨3���ڵĹؼ����ѶȽϴ�

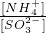 ______2���������������=����
______2���������������=����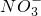 ��ԭΪNO��д���ù����в���NO��Ӧ�����ӷ���ʽ______��
��ԭΪNO��д���ù����в���NO��Ӧ�����ӷ���ʽ______��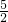
 ��5CO��g��+I2O5��s��=5 CO2��g��+I2��s������H=��-566.0kJ?mol-1����
��5CO��g��+I2O5��s��=5 CO2��g��+I2��s������H=��-566.0kJ?mol-1����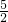 -��-75.56kJ?mol-1����
-��-75.56kJ?mol-1���� =-1377.22kJ/mol��
=-1377.22kJ/mol��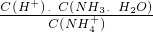 =
=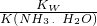 ��
�� =
=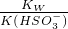 ��
��


 ��2011?ɽ�����о�NO2��SO2��CO�ȴ�����Ⱦ����Ĵ�������Ҫ���壮
��2011?ɽ�����о�NO2��SO2��CO�ȴ�����Ⱦ����Ĵ�������Ҫ���壮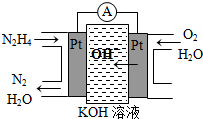 ���£�N2H4���ֳ��������㷺���ڻ���ƽ������л��ϳɼ�ȼ�ϵ�أ�NO2�Ķ�����N2O4���ǻ���г������������Իش���������
���£�N2H4���ֳ��������㷺���ڻ���ƽ������л��ϳɼ�ȼ�ϵ�أ�NO2�Ķ�����N2O4���ǻ���г������������Իش��������� �о�NO2��SO2��CO�ȴ�����Ⱦ����Ĵ���������Ҫ���壮
�о�NO2��SO2��CO�ȴ�����Ⱦ����Ĵ���������Ҫ���壮