
����Ŀ������þ��(Mg2B2O5��H2O����Fe2O3����)��ȡ����(H3BO3)������������¡�
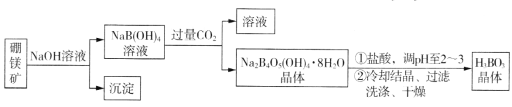
ͬ���������⣺
(1)��������Ҫ�ɷ�Ϊ____________________(�ѧʽ)��
(2)д������Na2B4O5(OH)4��8H2O�Ļ�ѧ����ʽ_________________________________��
(3)����H3BO3����ϴ�Ӹɾ��IJ�����______________________________��
(4)��֪��
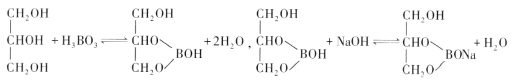
ʵ�������ô�ԭ���ⶨ������Ʒ�����������������ȷ��ȡ0.3000g��Ʒ����ƿ�У�����������ͼ���ʹ�����ܽⲢ��ȴ������1��2�η�̪��Һ��Ȼ����0.2000mol��L-1NaOH����Һ�ζ����յ㣬����NaOH��Һ22.00mL��
�ٵζ��յ������Ϊ________________________��
�ڸ�������Ʒ�Ĵ���Ϊ_________________��(����1λС��)��
(5)���NaB(OH)4��Һ�Ʊ�H3BO3�Ĺ���ԭ������ͼ��
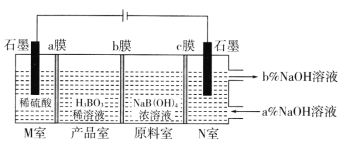
��bĤΪ________����Ĥ(������������������������)��������ÿ����1molH3BO3�������ҹ�����__________L����(��״��)��
��N���У����ںͳ���NaOH��Һ��Ũ�ȣ�a��_________b��(����>������<��)��
���𰸡�Mg(OH)2��Fe2O3 4NaB��OH��4+2CO2+3H2O=Na2B4O5��OH��48H2O��+2NaHCO3 ȡ���һ��ϴ��Һ�������Թ��У��μ������ữ����������Һ��������������˵��ϴ�Ӹɾ� ��Һ����ɫ��Ϊdz��ɫ���Ұ�����ڲ���ɫ 90.9 ������ 16.8 <
��������
��þ��������������Һ��Ӧ�����˳�ȥ����Mg��OH��2��Fe2O3��NaB��OH��4��Һ��ͨ������Ķ�����̼���õ�Na2B4O5��OH��48H2O��ΪNaHCO3�����˷��룬�������������С�����ᣬ���ϸ��ֽⷴӦ��ǿ���������ԭ������������ܽ�Ƚ�С����Na2B4O5��OH��48H2O���������ᷴӦ�õ����ᣬ��ȴ�ᾧ�����ˡ�ϴ�ӡ�����õ�����(H3BO3)���塣
(1)þ���ӿ�������������þ���������������������������Ʒ�Ӧ��
(2)��Ӧ��NaB��OH��4����CO2��������Na2B4O5(OH)4��8H2O��̼�����ƣ�д����ѧ����ʽ��
(3)ȡ���һ��ϴ��Һ���������������飻
(4) �ٸ��ݷ�̪�����ɫ�����������жϣ�
�����ù�ϵʽ�����м��㣻
(5)M������������ʧ���ӣ������Ӿ���aĤ�����Ʒ�ң�aĤΪ�����ӽ���Ĥ��ԭ������B��OH��4-ͨ��bĤ�����Ʒ����M�ҽ����H+��Ӧ����H3BO3��bĤΪ�����ӽ���Ĥ��ԭ����Na+����cĤ����N�ң�cĤΪ�����ӽ���Ĥ��N�������ӵõ�����������������������Ũ��������M����������������ˮ��N�������������ƺ��������ݴ˷�����
(1) ��þ�����������Ʒ�Ӧ��þ��������������þ������Fe2O3�����������Ʒ�Ӧ����˳�������Ҫ�ɷ�ΪMg(OH)2��Fe2O3��
�𰸣�Mg(OH)2��Fe2O3
(2) ��Ӧ��NaB��OH��4����CO2��������Na2B4O5(OH)4��8H2O��̼�����ƣ���ѧ����ʽΪ4NaB��OH��4+2CO2+3H2O=Na2B4O5��OH��48H2O��+2NaHCO3��
�𰸣�4NaB��OH��4+2CO2+3H2O=Na2B4O5��OH��48H2O��+2NaHCO3
(3)����H3BO3����ϴ�Ӹɾ��IJ�����ȡ���һ��ϴ��Һ�������Թ��У��μ������ữ����������Һ��������������˵��ϴ�Ӹɾ���
�𰸣�ȡ���һ��ϴ��Һ�������Թ��У��μ������ữ����������Һ��������������˵��ϴ�Ӹɾ�
(4)�ٵζ��յ������Ϊ��Һ����ɫ��Ϊdz��ɫ���Ұ�����ڲ���ɫ��
�𰸣���Һ����ɫ��Ϊdz��ɫ���Ұ�����ڲ���ɫ
��H3BO3��NaOH
1mol 1mol
n��H3BO3��0.2000mol/L��22.00��10-3L
��n��H3BO3��=0.0044mol
m��H3BO3��= n��H3BO3����M��H3BO3��=0.0044mol��62g/mol=0.2728g
����Ϊ![]() ��100%=90.9%
��100%=90.9%
(5)M������������ʧ�������������������Ӿ���aĤ�����Ʒ�ң�aĤΪ�����ӽ���Ĥ��ԭ������B��OH��4��ͨ��bĤ�����Ʒ����M�ҽ����H+��Ӧ����H3BO3��bĤΪ�����ӽ���Ĥ��ԭ����Na+����cĤ����N�ң�cĤΪ�����ӽ���Ĥ��N�������ӵõ�����������������������Ũ��������
�������������֪bĤΪ�����ӽ���Ĥ����ΪH++ B��OH��4��=H3BO3+H2O�����ת��1mol��������1mol H3BO3���й�ϵʽ
1mole-��1/4O2��M�ң���1mol H3BO3��1/2H2��N�ң�
������ÿ����1molH3BO3�������ҹ����ɣ�1/2+1/4��mol��22.4L/mol=16.8L����(��״��)��
�������������֪N���У����ںͳ���NaOH��Һ��Ũ�ȣ�a��<b����
�𰸣������� 16.8 <


 �������Ӧ���⼯ѵϵ�д�
�������Ӧ���⼯ѵϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ʵ�����ý�����ȷ����

A. ������Һ��ɫ��ȥ��ԭ���ǣ�CH3COOC2H5 + NaOH =CH3COONa + C2H5OH
B. ������Һ����ԭ���ǣ�CH3COO�� + H2O ![]() CH3COOH + H+
CH3COOH + H+
C. ��ʵ��١��ڡ����Ʋ⣬���к�ɫ��ȥ��ԭ��������������ȡ�˷�̪
D. ���к�ɫ��ȥ֤���Ҳ�С�Թ����ռ��������������л�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����һ���¶��£���һ���ݻ��ɱ�������У�ͨ��3 mol SO2��2 mol O2�����������ʹ֮��Ӧ��2SO2(g)��O2(g) ![]() 2SO3(g)����H����196.6 kJ��mol��1��ƽ��ʱ����������ѹǿΪ��ʼʱ��90%������ͬһ��Ӧ�¶ȣ�����ͬ�����У�����ʼ���ʵ�����Ϊ4 mol SO2��3 mol O2��2 mol SO3(g)������˵����ȷ����(����)
2SO3(g)����H����196.6 kJ��mol��1��ƽ��ʱ����������ѹǿΪ��ʼʱ��90%������ͬһ��Ӧ�¶ȣ�����ͬ�����У�����ʼ���ʵ�����Ϊ4 mol SO2��3 mol O2��2 mol SO3(g)������˵����ȷ����(����)
A. ��һ��ƽ��ʱ��Ӧ�ų�������Ϊ294.9 kJ
B. ����ƽ��SO2��ת�������
C. �ڶ��δ�ƽ��ʱSO3�������������![]()
D. ��ƽ��ʱ��O2��ʾ�ķ�Ӧ����Ϊ0.25 mol��(L��min)��1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����B��A��뵼�����ײ��ϣ���CdTe��CdSe��ZnSe��ZnS�ȣ��ڹ����������̫���ܵ���Լ�����̽��ȷ����й�����ǰ�����ش��������⣺
��1����̬п��Zn��ԭ�ӵĵ����Ų�ʽΪ[Ar]_____��
��2�������ܼ�������ɵĵ��������Ǹ��ܼ�ԭ�ӹ�����Ķ�������֧����һ���۵�������______�����ţ�
a ����ԭ�� b ����ԭ�� c ���ع��� d �������ԭ��
��3�������ڱ��У�Se��As��Brͬ�������ڣ���S��Teͬ�������ڡ�Te��As��Se��Br�ĵ�һ�������ɴ�С����Ϊ_______��
��4��H2O2��H2S����Է���������ȣ������£�H2O2��Һ̬����H2S����̬������Ҫԭ����______��![]() ������ԭ���ӻ�����Ϊ_______����ռ乹��Ϊ_______��
������ԭ���ӻ�����Ϊ_______����ռ乹��Ϊ_______��
��5��ZnO���ж��صĵ�ѧ����ѧ���ԣ���һ��Ӧ�ù㷺�Ĺ��ܲ��ϡ�
����֪пԪ�ء���Ԫ�صĵ縺�Էֱ�Ϊ1.65��3.5��ZnO�л�ѧ��������Ϊ______��ZnO���Ա�NaOH��Һ�ܽ�����[Zn(OH)4]2������ӻ�ѧ���ǶȽ����ܹ��γɸ����ӵ�ԭ��_______��

��һ��ZnO����ľ�����ͼ��ʾ�������߳�Ϊa nm�������ӵ�������ֵΪNA���侧���ܶ�Ϊ________g��cm3��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������Ũ�Ⱦ�Ϊ0.1 mol��L��1��������Һ�������ᡢ�ڴ��ᡢ���������ơ����Ȼ�李��ݴ���李���������李��߰�ˮ����ش��������⣺
��1���١��ڡ��ۡ���������Һ����ˮ�������H��Ũ���ɴ�С��˳����(�����)___________��
��2���ܡ��ݡ��ޡ���������Һ��NH![]() Ũ���ɴ�С��˳����(�����)_______________��
Ũ���ɴ�С��˳����(�����)_______________��
��3�����ۺܰ͢������1��2��Ϻ��Һ�и�����Ũ���ɴ�С��˳���ǣ�__________________��
��4����֪t ��ʱ��KW��1��10��13����t ��(�����������������)________25�档��t ��ʱ��pH��11��NaOH��Һa L��pH��1��H2SO4��Һb L���(���Ի�Ϻ���Һ����ı仯)�������û����Һ��pH��2����a��b��________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����NAΪ�����ӵ�������ֵ������˵����ȷ����
A. 22.4LCl2��������ˮ��������Һ��Cl2��Cl-��HClO��ClO-����������ΪNA
B. ��״���£�38g3H2O2�к���3NA���ۼ�
C. �����£���5.6g����Ͷ������Ũ�����У�ת��0.3NA����
D. 0.1molL-1MgCl2��Һ�к��е�Mg2+��Ŀһ��С��0.1NA
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ijɱ��ҩ��M�ĺϳ�·������ͼ��ʾ��

�ش��������⣺
(1)A�й����ŵ�������_______________��B��C�ķ�Ӧ������__________________��
(2)B�ķ���ʽΪ________________________��
(3)C��D�Ļ�ѧ����ʽΪ__________________________��
(4)F�Ľṹ��ʽΪ___________________________��
(5)��������������C��ͬ���칹�干��____________��(�����������칹)��
���ܷ���ˮ�ⷴӦ��������FeCl3��Һ������ɫ��Ӧ��
���к˴Ź�������Ϊ4���Ľṹ��ʽΪ_______________(��дһ��)��
(6)����![]() ��CH3CH2OHΪԭ�ϣ�����Ʊ��л�������
��CH3CH2OHΪԭ�ϣ�����Ʊ��л������� �ĺϳ�·��(���Լ���ѡ)_______________��
�ĺϳ�·��(���Լ���ѡ)_______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������������������о���ʮ����Ҫ�����ã���������ӵ����̫���ܵ��ռ�кܴ���ء�̫���ܵ����ͨ�����ЧӦ���߹⻯ѧЧӦֱ�Ӱѹ���ת���ɵ��ܵ�װ�á�������е����裬����ͭ���ࡢ�ء����Ȼ����
��1����̬��ͭ�����е���ռ�ݵ�ԭ�ӹ����ĿΪ____________��
��2������̬��ԭ�Ӽ۲�����Ų�ʽд��4s24px24py2������Υ����____________��
��3����ͼ��ʾ̼�������Ԫ�ص��ļ������ܱ仯���ƣ����б�ʾ��������_______(����)��

��4��Ԫ��X���ͬ������ԭ�Ӱ뾶��С��X�γɵ�����⻯��Q�ĵ���ʽΪ_____���÷���������ԭ�ӵ��ӻ�����Ϊ_____��д��һ����Q��Ϊ�ȵ����������______��
��5������Ԫ�ش���ͬһ�������Ԫ�ؾ���ȱ�����ԡ���Ȼ���к���Ԫ�ص�������һ����Ȼ��أ��仯ѧʽд��Na2B4O7��10H2O��ʵ�������Ľṹ��Ԫ��������H3BO3������[B(OH)4]-���϶��ɵ�˫��Ԫ����Ӧ��д��Na2[B4O5(OH)4]8H2O����ṹ��ͼ��ʾ�����������ӿ��γ���״�ṹ����þ����в����ڵ���������__________(��ѡ����ĸ)��

A ���Ӽ� B ���ۼ� C ������ D ���»��� E ���
��6��GaAs���۵�Ϊ1238�棬�ܶ�Ϊ��g��cm3���侧���ṹ��ͼ��ʾ����֪GaAs��GaN������ͬ�ľ����ṹ������߾�������;�Ϊ____��GaAs���۵�____������������������������GaN��Ga��As��Ħ�������ֱ�ΪMGa gmol1��MAs gmol1��ԭ�Ӱ뾶�ֱ�ΪrGa pm��rAs pm�������ӵ�����ֵΪNA����GaAs������ԭ�ӵ����ռ��������İٷ���Ϊ_______��

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ʳƷ��ȫһֱ������ע�Ļ��⡣������������(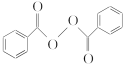 )��ȥ�����������������Ŀǰ�ѱ����á��ϳɹ�����������������ͼ���£�
)��ȥ�����������������Ŀǰ�ѱ����á��ϳɹ�����������������ͼ���£�
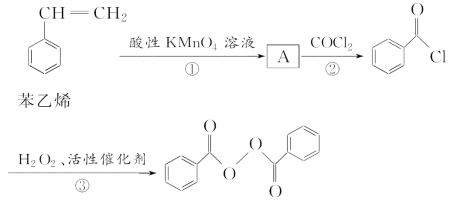
��ش��������⣺
��1��A�Ľṹ��ʽΪ________________���ڵķ�Ӧ����Ϊ________________��
��2��д����Ӧ�۵Ļ�ѧ����ʽ��_________________________________________
������������������������ˮ��Ļ�ѧ����ʽΪ__________________________________��
��3�������йر���ϩ��˵����ȷ����______(����ĸ)��
A������ϩ��ʹ��ˮ��ɫ B������ϩ����һ��ͬ���칹�壬��һ�ȴ������һ��
C������ϩ������8��̼ԭ�ӿ��ܹ�ƽ�� D������ϩ������ȼ�պ������϶����
��4��д��һ����������Ҫ��Ĺ�������������ͬ���칹��Ľṹ��ʽ______________��
�ٷ����в���̼̼˫����������
�ڷ�����ֻ����һ�ֺ��������ţ�
�ۺ˴Ź���������3��壬������֮��Ϊ1��2��2��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com