
(13��) ��ͼ����ѧ��ѧ�г������ʵ�ת����ϵ���������ʺͷ�Ӧ������ȥ��
����֪��2Al
+ 3FeO 3Fe + Al2O3��2Al
+ Fe2O3
3Fe + Al2O3��2Al
+ Fe2O3 2Fe + Al2O3��
2Fe + Al2O3��
8Al + 3Fe3O4 9Fe + 4Al2O3��
9Fe + 4Al2O3��
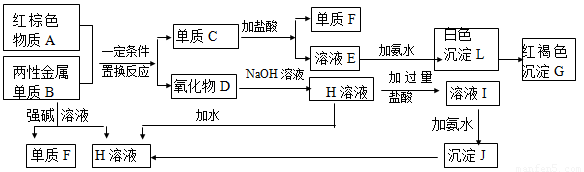
(1)������A�Ļ�ѧʽ�� �� д������A��һ����; ��
(2)д������B��ǿ����Һ��Ӧ�����ӷ���ʽ ��
(3)д���ɳ���J���� H��Һ�����ӷ���ʽ ��
(4)��ҺE�м��백ˮʱ�������ɰ�ɫ����L��д������L�����ӷ���ʽ ��
��ɫ����L��Ѹ�ٱ�Ϊ ɫ�����ձ�Ϊ���ɫ����G��д��L��ΪG�Ļ�ѧ��Ӧ����ʽ ��
(5)��ҺI����������������_____________________��
 �Ż���ҵ�Ϻ��Ƽ����׳�����ϵ�д�
�Ż���ҵ�Ϻ��Ƽ����׳�����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(13��) ��ͼ��ʵ�������Ʊ��������֤�������ʵ�װ��ͼ
��1������a��������_________________��
��2��������ͼװ���Ʊ������������������
��Բ����ƿ�ڷ�����Ӧ�Ļ�ѧ����ʽΪ____________________________________��
��װ��B�е���ҺΪ_________________���ձ�����Һ������Ϊ____________________ ��
��Eװ�÷�����Ӧ�����ӷ���ʽΪ____________________________________��
��3������ͼ��ʾԲ����ƿ�ڼ���̼��a�м���Ũ���ᣬ��ʼʵ�飬���Ȳ��������建��ͨ������װ���������ʵ�飺
ʵ��1��֤��SO2����Ư���Ժͻ�ԭ��
ʵ��2��֤��̼Ԫ�صķǽ����Աȹ�Ԫ�ص�ǿ
��B��Ϊ����Ʒ����Һ��C��Ϊ��������KMnO4��Һ,��֤��SO2����Ư���Ե�����Ϊ__________________________________________��
��D��Ӧ����������____________������Һ���ƣ���E�м���____________������Һ���ƣ���֤��̼Ԫ�صķǽ����Աȹ�Ԫ�ص�ǿ������Ϊ___________________________��
��Ϊ���ʵ��2��ȷ�ԣ�����Ľ���װ��Ϊ___________________������װ�ô��ţ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(13��) ��ͼ��ʵ�������Ʊ��������֤�������ʵ�װ��ͼ
��1������a��������_______________________��
��2��������ͼװ���Ʊ������������������
��Բ����ƿ�ڷ�����Ӧ�����ӷ���ʽΪ____________________________________________��
��װ��B�е���ҺΪ________________________���ձ�����Һ������Ϊ__________��
��3������ͼ��ʾԲ����ƿ�ڼ���̼��a�м���Ũ���ᣬ��ʼʵ�飬���Ȳ��������建��ͨ������װ���������ʵ�飺
ʵ��1��֤��SO2����Ư���Ժͻ�ԭ��
ʵ��2��֤��̼Ԫ�صķǽ����Աȹ�Ԫ�ص�ǿ
��B��Ϊ����Ʒ����Һ��C��Ϊ��������KMnO4��Һ,��֤��SO2����Ư���Ե�����Ϊ__________��
��D��Ӧ����������____________������Һ���ƣ���E�м���____________������Һ���ƣ���֤��̼Ԫ�صķǽ����Աȹ�Ԫ�ص�ǿ������Ϊ________________________________________��
��Ϊ���ʵ��2��ȷ�ԣ�����Ľ���װ��Ϊ___________________������װ�ô��ţ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(13��) ��ͼ����ѧ��ѧ�г������ʵ�ת����ϵ���������ʺͷ�Ӧ������ȥ��
����֪��2Al+ 3FeO![]() 3Fe + Al2O3��2Al+ Fe2O3
3Fe + Al2O3��2Al+ Fe2O3 2Fe + Al2O3��
2Fe + Al2O3��
8Al + 3Fe3O4![]() 9Fe + 4Al2O3��
9Fe + 4Al2O3��
(1)������A�Ļ�ѧʽ�� ��д������A��һ����; ��
(2)д������B��ǿ����Һ��Ӧ�����ӷ���ʽ ��
(3)д���ɳ���J���� H��Һ�����ӷ���ʽ ��
(4)��ҺE�м��백ˮʱ�������ɰ�ɫ����L��д������L�����ӷ���ʽ ��
��ɫ����L��Ѹ�ٱ�Ϊ ɫ�����ձ�Ϊ���ɫ����G��д��L��ΪG�Ļ�ѧ��Ӧ����ʽ ��
(5)��ҺI����������������_____________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012��ɽ��ʡ��Ƿ�����ѧ������ѧ�ڼ���ѧϰЧ����⿼�Ի�ѧ�Ծ� ���ͣ�ʵ����
(13��) ��ͼ��ʵ�������Ʊ�������� ֤�������ʵ�װ��ͼ
֤�������ʵ�װ��ͼ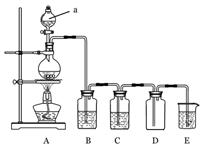
��1������a��������_________________��
��2��������ͼװ���Ʊ������������������
��Բ����ƿ�ڷ�����Ӧ�Ļ�ѧ����ʽΪ____________________________________��
��װ��B�е���ҺΪ_________________���ձ�����Һ������Ϊ____________________  ��
��
��Eװ�÷�����Ӧ�����ӷ���ʽΪ____________________________________��
��3������ͼ��ʾԲ����ƿ�ڼ���̼��a�м���Ũ���ᣬ��ʼʵ�飬���Ȳ��������建��ͨ������װ���������ʵ�飺
ʵ��1��֤��SO2����Ư���Ժͻ�ԭ��
ʵ��2��֤��̼Ԫ�صķǽ����Աȹ�Ԫ�ص�ǿ
��B��Ϊ����Ʒ����Һ��C��Ϊ��������KMnO4��Һ,��֤��SO2����Ư���Ե�����Ϊ__________________________________________��
��D��Ӧ����������____________������Һ���ƣ���E�м���____________������Һ���ƣ���֤��̼Ԫ�صķǽ����Աȹ�Ԫ�ص�ǿ������Ϊ___________________________��
��Ϊ���ʵ��2��ȷ�ԣ�����Ľ���װ��Ϊ___________________������װ�ô��ţ�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com