
ij����С���������ͼ��ʾ��װ�ã����ڻ��������������Ƶ���ǿ�����е������������л������NaCl��Һ�����ʵ�飨��ʱ����ֹˮ��a���ر�ֹˮ��b�������ڴ��ģ�ʵ�鲢δ�ﵽԤ��Ŀ�ģ���Ҳ���������˺ܸ��˵�����(�����ӽ���Ĥֻ���������Ӻ�ˮ��ͨ��)����������Ƿ������ش��������⣺
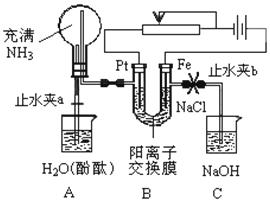
(1)д��Bװ���еĵ缫��Ӧ��
������____________ ������____________
(2)�ش�۲쵽Aװ���е�����
��_________ ��
����ƿ��Һ������������������һ���̶Ⱥ������������������ƽ��
��________ ��
(3)���۲쵽Aװ���е���������ǹر�ֹˮ��a����ֹˮ��b���ٹ۲�Cװ�ã���������˵�����ɣ�����������д���йط�Ӧ�Ļ�ѧ����ʽ(�����ӷ�Ӧ��д���ӷ���ʽ)��
(4)����ﵽ���NaCl��Һ��Ŀ�ģ�Ӧ��θĽ�װ�ã��������������
_______________________________________________________________________��
(1)2H2O+2e��==2OH��+ H2��(��2H+ + 2e��==H2��) (1��)��Fe��2e��== Fe2+ (1��)
(2)��A�ձ��е�H2O������������ɫ��Ȫ ��2��)
�����A�ձ���Һ�ʺ�ɫ��������������� (2��)
(3) Fe2+ +2OH��== Fe(OH)2����4Fe(OH)2 + 2H2O + O2== 4Fe(OH)3
(��4Fe2+ + 8OH��+ 2H2O + O2== 4Fe(OH)3��) (2��)
(4)��Fe�缫����C��Pt�ȶ��Ե缫��װ�������缫����λ�õ� (1��)


 ���Ͱ�ͨ��ĩ���ϵ�д�
���Ͱ�ͨ��ĩ���ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
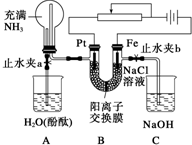 ij����С���������ͼ��ʾ��װ�ã����ڻ��������������Ƶ���ǿ�����е������������л������NaCl��Һ�����ʵ�飨��ʱ����ֹˮ��a���ر�ֹˮ��b�������ڴ��ģ�ʵ�鲢δ�ﵽԤ��Ŀ�ģ���Ҳ���������˺ܸ��˵����������ӽ���Ĥֻ���������Ӻ�ˮͨ������
ij����С���������ͼ��ʾ��װ�ã����ڻ��������������Ƶ���ǿ�����е������������л������NaCl��Һ�����ʵ�飨��ʱ����ֹˮ��a���ر�ֹˮ��b�������ڴ��ģ�ʵ�鲢δ�ﵽԤ��Ŀ�ģ���Ҳ���������˺ܸ��˵����������ӽ���Ĥֻ���������Ӻ�ˮͨ�������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
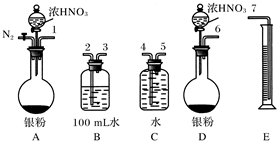
| 2 | 3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
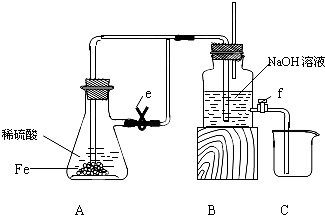
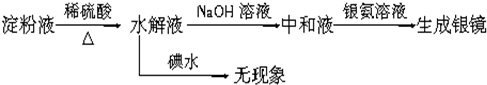
| ϡ���� |
| �� |
| NaOH��Һ |
| ��ˮ |
| ϡ���� |
| �� |
| ������Һ |
| �� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com