
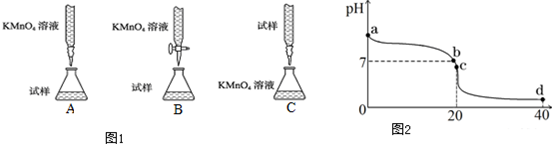
���� ��1���ٸ�����ؾ���ǿ�����ԣ��������ܣ������ü�ʽ�ζ��ܣ�
��������Һ�IJ����У����㡢�������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȵȣ��������Ʋ����ж�ʹ�õ�������Ȼ��ȷ����ȱ�ٵ�������
��2����һˮ�ϰ���������ʣ���ˮ��Һ����ڵ���ƽ�⣬�������ӻ���������õ�ˮ�������������Ũ�ȣ�
��pH=7ʱ����Һ��c��H+��=c��OH-������Һ�д��ڵ���غ㣬�ٽ�ϵ���غ��ж�c��Cl-����c��NH4+������Դ�С��
��c��ʱ������ǡ�÷�Ӧ�����Ȼ�泥��Ȼ����ǿ�������Σ�笠�����ˮ���ʹ����Һ�����ԣ�
��d��ʱ����Һ�е�����Ϊ�����ʵ���Ũ�ȵ��Ȼ�狀����ᣬ��Һ�����ԣ��Ȼ�����ȫ���룬笠�������ˮ�⣬�ݴ��ж���Һ������Ũ�ȴ�С��
��� �⣺��1����������KMnO4��Һ�ζ�ij��Ѫ����������ؾ���ǿ�����ԣ��������ܣ������ü�ʽ�ζ��ܣ�����B���ϣ�
��ѡ��B��
������һ�����ʵ���Ũ�ȵ�����KMnO4��Һ250mL�IJ���Ϊ�����㡢�������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȵȲ�����һ����������ƽ��������ҩ��ȡ��ҩƷ�����ձ����ܽ⣨������Ͳ��ȡˮ������ȴ��ת�Ƶ�250mL����ƿ�У����ò�����������ϴ���ձ���������2��3�Σ���ϴ��Һ��������ƿ����ˮ��Һ�����̶���1��2cmʱ�����ý�ͷ�ιܵμӣ�������Ҫ������Ϊ������ƽ��ҩ�ס����������ձ�����Ͳ�����ÿɲ��ã���250mL����ƿ����ͷ�ιܣ�������ʹ�õIJ�������Ϊ��250mL����ƿ��
�ʴ�Ϊ��250mL����ƿ��
��2����һˮ�ϰ���������ʣ���ˮ��Һ��ֻ�в��ֵ��룬��������������Ӻ�笠����ӣ�һˮ�ϰ��ĵ��뷽��ʽΪ��NH3•H2O?NH4++OH-�����ӻ�����=c��H+��c��OH-����������Һ��pH=10����ˮ�������������Ũ��Ϊ10-10mol/L��
�ʴ�Ϊ��10-10mol/L��
��b��ʱpH=7������Һ��cc��H+��=c��OH-������Һ�д��ڵ���غ㣬���ݵ���غ��c��Cl-��+c��OH-��=c��NH4+��+cc��H+�������Ե�c��Cl-��=c��NH4+�����ʴ�Ϊ��=��
��c��ʱ����ǡ�÷�Ӧ�����Ȼ�泥��Ȼ����ǿ�������Σ�笠�����ˮ���ʹ��Һ��������Ũ�ȴ�������������Ũ�ȣ�����Һ�����ԣ�ˮ�����ӷ���ʽΪ��NH4++H2O?NH3•H2O+H+��ȡc��ʱ�ı�����Һ���������ˮ���������������ˮ���Լ��ԣ�笠����Ӻ��������ˮ����ٽ����ɰ�ɫ���������һˮ�ϰ������ӷ���ʽΪ��SiO32-+2NH4++H2O=H2SiO3��+2NH3•H2O��
�ʴ�Ϊ��笠����Ӻ��������ˮ����ٽ����ɰ�ɫ���������һˮ�ϰ������ӷ���ʽΪ��SiO32-+2NH4++H2O=H2SiO3��+2NH3•H2O��
��d��ʱ��������ʵ����ǰ�ˮ��2���������ʱ����Һ�е�����Ϊ�����ʵ���Ũ�ȵ��Ȼ�狀����ᣬ��Һ�����ԣ��Ȼ�����ȫ���룬笠�����ˮ�ˮ��̶Ƚ�С����������غ�֪����Һ������Ũ�ȴ�С˳����c ��Cl-����c ��H+����c ��NH4+����c ��OH-����
�ʴ�Ϊ��c��Cl-����c��H+����c��NH4+����c��OH-����
���� ���⿼�����к͵ζ�����������ԭ�ζ��������ؿ���ѧ���������жϼ�ʵ�������������ȷ�ζ�ԭ����������ʵ����֪ʶ���ǽⱾ��ؼ�����Ŀ�Ѷ��еȣ�


 ��Ȥ������ҵ���ϿƼ�������ϵ�д�
��Ȥ������ҵ���ϿƼ�������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��Ȼ����������ȼ�� | B�� | ���ƾ�����ҽ������ | ||
| C�� | ����ˮ������ɱ���� | D�� | �����������ڽ�������걾 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| Na2S2O3��ʼ���� | Na2S2O3 �յ���� | |
| ��һ�� | 0.10mL | 18.30mL |
| �ڶ��� | 0.30mL | 18.30mL |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Na+�ĵ����Ų�ͼ�� | B�� | Na+�Ľṹʾ��ͼ��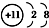 | ||
| C�� | Na�ĵ����Ų�ʽ��1s22s22p63s1 | D�� | Na�ļ����Ų�ʽ��[Ne]3s1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
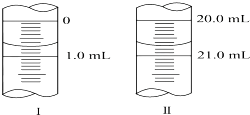 �ڷ�����ѧ�г���Na2C2O4������Ϊ�����ʲⶨKMnO4��Һ��Ũ�ȣ���H2SO4��Һ�У���Ӧ���£�
�ڷ�����ѧ�г���Na2C2O4������Ϊ�����ʲⶨKMnO4��Һ��Ũ�ȣ���H2SO4��Һ�У���Ӧ���£��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �� | B�� | �մ� | C�� | ���� | D�� | �������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Na2CO3Ũ�ȼ�С | B�� | Na2CO3Ũ������ | ||
| C�� | Na2CO3Ũ�������о������� | D�� | Na2CO3Ũ�Ȳ��䣬���о������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com