
 N2O4(g) ��H=-52.7kJ��mol-1
N2O4(g) ��H=-52.7kJ��mol-1 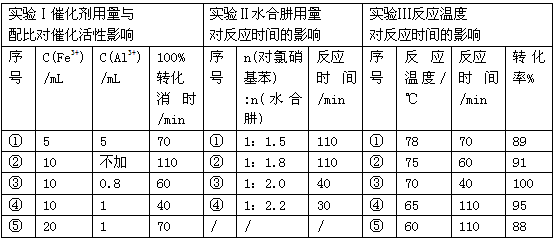
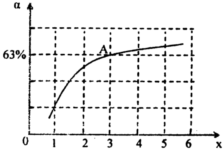
 N2O4��g���ﵽƽ�⡣������������ʱ�����д�ʩ�����NO2ת���ʵ���__________������ĸ��
N2O4��g���ﵽƽ�⡣������������ʱ�����д�ʩ�����NO2ת���ʵ���__________������ĸ�� N2O4��g����ƽ�ⳣ��K��
N2O4��g����ƽ�ⳣ��K��  �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 �£�N2H4���ֳ��������㷺���ڻ���ƽ������л��ϳɼ�ȼ�ϵ�أ�NO2�Ķ�����N2O4���ǻ���г��õ�����������ش��������⣺
�£�N2H4���ֳ��������㷺���ڻ���ƽ������л��ϳɼ�ȼ�ϵ�أ�NO2�Ķ�����N2O4���ǻ���г��õ�����������ش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
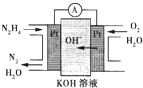
 ��2013?������һģ���£�N2H4���Ͱ��ǵ������ֳ���������ڿ�ѧ�������������й㷺Ӧ�ã��밴Ҫ��ش��������⣺
��2013?������һģ���£�N2H4���Ͱ��ǵ������ֳ���������ڿ�ѧ�������������й㷺Ӧ�ã��밴Ҫ��ش��������⣺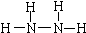
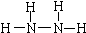
| ||
| ||
| T/�� | 165 | 175 | 185 | 195 |
| K | 111.9 | 74.1 | 50.6 | 34.8 |
| n(NH3) |
| n(CO2) |
| n��N2�� | n��H2�� | n��NH3�� | |
| �� | 1mol | 3mol | 0mol |
| �� | 0.5mol | 1.5mol | 1mol |
| �� | 0mol | 0mol | 4mol |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
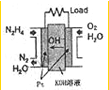 �£�N2H4���㷺���ڻ���ƽ������л��ϳɼ����ȼ�ϣ��Ȼش���������
�£�N2H4���㷺���ڻ���ƽ������л��ϳɼ����ȼ�ϣ��Ȼش���������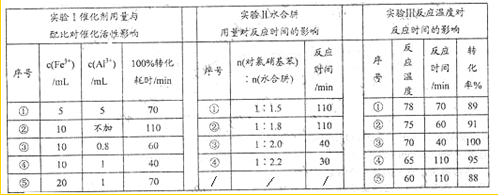
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ģ���� ���ͣ������
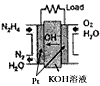
 N2O4(g) ��H=-52��7kJ��mol-1 ��д����̬������̬������������ȼ�����ɵ�������̬ˮ���Ȼ�ѧ����ʽ��__________________________��
N2O4(g) ��H=-52��7kJ��mol-1 ��д����̬������̬������������ȼ�����ɵ�������̬ˮ���Ȼ�ѧ����ʽ��__________________________�� 
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com