
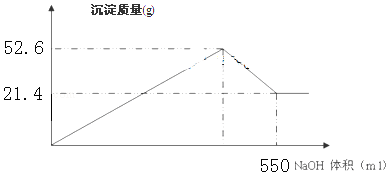
·ÖĪö £Ø1£©ĀČ»ÆĢśÓėĒāŃõ»ÆÄĘ·“Ӧɜ³ÉĒāŃõ»ÆĢś³ĮµķÓėĀČ»ÆÄĘ£¬ĀČ»ÆĀĮÓė¹żĮæµÄĒāŃõ»ÆÄĘČÜŅŗ·“Ӧɜ³ÉĘ«ĀĮĖįÄĘÓėĖ®£»
£Ø2£©×īÖÕ³Įµķ21.4gĪŖFe£ØOH£©3£¬¼ÓČČ·Ö½āÉś³ÉFe2O3£¬øł¾Żn=$\frac{m}{M}$¼ĘĖćĒāŃõ»ÆĢśĪļÖŹµÄĮ棬øł¾ŻFeŌŖĖŲŹŲŗć¼ĘĖćn£ØFe2O3£©£¬ŌŁøł¾Żm=nM¼ĘĖćm£ØFe2O3£©£»
£Ø3£©øł¾ŻFeŌŖĖŲŹŲŗć¼ĘĖćn£ØFeCl3£©=n[Fe£ØOH£©3]£¬ŌŁøł¾Żc=$\frac{n}{V}$¼ĘĖćc£ØFeCl3£©£»
£Ø4£©³Įµķ×ī“óŹ±ĪŖ52.6g£¬ĪŖĒāŃõ»ÆĀĮ”¢ĒāŃõ»ÆĢśµÄÖŹĮæÖ®ŗĶ£¬¼ĘĖćĒāŃõ»ÆĀĮµÄÖŹĮ棬øł¾Żn=$\frac{m}{M}$¼ĘĖćĒāŃõ»ÆĀĮµÄĪļÖŹµÄĮ棬øł¾ŻAlŌŖĖŲŹŲŗć¼ĘĖćn£ØAlCl3£©£¬ŌŁøł¾ŻŌŁøł¾Żc=$\frac{n}{V}$¼ĘĖćc£ØAlCl3£©£»
£Ø5£©¼ÓČė550mL NaOHČÜŅŗ³ĮŹ±£¬ČÜŅŗÖŠČÜÖŹĪŖNaCl”¢NaAlO2£¬øł¾ŻĀČĄė×ÓŹŲŗćn£ØNaCl£©=3n£ØFeCl3£©+3n£ØAlCl3£©£¬øł¾ŻAlŌŖĖŲŹŲŗćn£ØNaAlO2£©=n£ØAlCl3£©£¬øł¾ŻÄĘĄė×ÓŹŲŗćn£ØNaOH£©=n£ØNaCl£©+n£ØNaAlO2£©£¬ŌŁøł¾Żc=$\frac{n}{V}$¼ĘĖćc£ØNaOH£©£®
½ā“š ½ā£ŗ£Ø1£©ĀČ»ÆĢśÓėĒāŃõ»ÆÄĘ·“Ӧɜ³ÉĒāŃõ»ÆĢś³ĮµķÓėĀČ»ÆÄĘ£¬·“Ó¦Ąė×Ó·½³ĢŹ½ĪŖ£ŗFe3++3OH-=Fe£ØOH£©3”ż£¬ĀČ»ÆĀĮÓė¹żĮæµÄĒāŃõ»ÆÄĘČÜŅŗ·“Ӧɜ³ÉĘ«ĀĮĖįÄĘÓėĖ®£¬·“Ó¦Ąė×Ó·½³ĢŹ½ĪŖAl3++4OH-=AlO2-+2H2O£¬
¹Ź“š°øĪŖ£ŗFe3++3OH-=Fe£ØOH£©3”ż£»Al3++4OH-=AlO2-+2H2O£»
£Ø2£©×īÖÕ³Įµķ21.4gĪŖFe£ØOH£©3£¬¼ÓČČ·Ö½āÉś³ÉFe2O3£¬n[Fe£ØOH£©3]=$\frac{21.4g}{107g/mol}$=0.2mol£¬øł¾ŻFeŌŖĖŲŹŲŗćn£ØFe2O3£©=$\frac{0.2mol}{2}$=0.1mol£¬¹Źm£ØFe2O3£©=0.1mol”Į160g/mol=16g£¬
¹Ź“š°øĪŖ£ŗ16£»
£Ø3£©øł¾ŻFeŌŖĖŲŹŲŗćn£ØFeCl3£©=n[Fe£ØOH£©3]=0.1mol£¬Ōņc£ØFeCl3£©=$\frac{0.2mol}{0.2L}$=1mol/L£¬
¹Ź“š°øĪŖ£ŗ1£»
£Ø4£©³Įµķ×ī“óŹ±ĪŖ52.6g£¬ĪŖĒāŃõ»ÆĀĮ”¢ĒāŃõ»ÆĢśµÄÖŹĮæÖ®ŗĶ£¬ĒāŃõ»ÆĀĮµÄÖŹĮæĪŖ52.6g-21.4g=31.2g£¬ĒāŃõ»ÆĀĮµÄĪļÖŹµÄĮæĪŖ$\frac{31.2g}{78g/mol}$=0.4mol£¬øł¾ŻAlŌŖĖŲŹŲŗćn£ØAlCl3£©=0.4mol£¬c£ØAlCl3£©=$\frac{0.4mol}{0.2L}$=2mol/L£¬
¹Ź“š°øĪŖ£ŗ2£»
£Ø5£©¼ÓČė550mL NaOHČÜŅŗ³ĮŹ±£¬ČÜŅŗÖŠČÜÖŹĪŖNaCl”¢NaAlO2£¬øł¾ŻĀČĄė×ÓŹŲŗćn£ØNaCl£©=3n£ØFeCl3£©+3n£ØAlCl3£©=3”Į0.2mol+3”Į0.4mol=1.8mol£¬øł¾ŻAlŌŖĖŲŹŲŗćn£ØNaAlO2£©=n£ØAlCl3£©=0.4mol£¬øł¾ŻÄĘĄė×ÓŹŲŗćn£ØNaOH£©=n£ØNaCl£©+n£ØNaAlO2£©=1.8mol+0.4mol=2.2mol£¬¹Źc£ØNaOH£©=$\frac{2.2mol}{0.55L}$=4mol/L£¬
¹Ź“š°øĪŖ£ŗ4£®
µćĘĄ ±¾ĢāŅŌ»Æѧ·“Ó¦Ķ¼ĻóŠĪŹ½£¬æ¼²é»ģŗĻĪļ¼ĘĖć£¬Ć÷Č·ø÷½×¶Ī·¢ÉśµÄ·“Ó¦ŹĒ¹Ų¼ü£¬×¢ŅāĄūÓĆŹŲŗć·Ø½ųŠŠ½ā“š£¬ÄѶČÖŠµČ£®



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | Fe3++S2-=Fe2++S | B£® | Fe3++Fe=2Fe2+ | ||
| C£® | Fe2++Cl2=Fe3++2Cl- | D£® | 2Fe3++2I-=2Fe2++I2 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ĄūÓƶ”“ļ¶ūŠ§Ó¦æÉĒų·Öµ°°×ÖŹČÜŅŗÓėĘĻĢŃĢĒČÜŅŗ | |
| B£® | ½«±„ŗĶFeCl3ČÜŅŗµĪČė·ŠĖ®ÖŠ£¬½«¼ĢŠųÖó·ŠÖĮČÜŅŗ³ŹŗģŗÖÉ«¼“Éś³ÉĒāŃõ»ÆĢś½ŗĢå | |
| C£® | ÓĆ¹żĀĖ·ØæÉŅŌ³żČ„Fe£ØOH£©3½ŗĢåÖŠµÄFeCl3 | |
| D£® | Óƶ¹½¬ÖŠ¼ÓČėĮņĖįøĘÖʶ¹øÆ£¬ŹĒĄūÓĆĮĖ½ŗĢåµÄ¾Ū³ĮŠŌÖŹ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
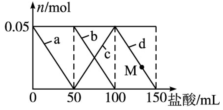 Ļņ100mLŗ¬Na2CO3”¢NaAlO2µÄ»ģŗĻČÜŅŗÖŠÖšµĪ¼ÓČė150mL 1mol•L-1HClČÜŅŗ£¬²āµĆČÜŅŗÖŠµÄij¼øÖÖĄė×ÓĪļÖŹµÄĮæµÄ±ä»ÆČēĶ¼ĖłŹ¾£¬ŌņĻĀĮŠĖµ·ØÕżČ·µÄŹĒ£Ø””””£©
Ļņ100mLŗ¬Na2CO3”¢NaAlO2µÄ»ģŗĻČÜŅŗÖŠÖšµĪ¼ÓČė150mL 1mol•L-1HClČÜŅŗ£¬²āµĆČÜŅŗÖŠµÄij¼øÖÖĄė×ÓĪļÖŹµÄĮæµÄ±ä»ÆČēĶ¼ĖłŹ¾£¬ŌņĻĀĮŠĖµ·ØÕżČ·µÄŹĒ£Ø””””£©| A£® | aĒśĻß±ķŹ¾µÄĄė×Ó·½³ĢŹ½ĪŖ£ŗAlO2-+H++H2OØTAl£ØOH£©3”ż | |
| B£® | bĒśĻß±ķŹ¾Ģ¼ĖįÄĘŗĶŃĪĖį·“Ó¦£¬dĒśĻß±ķŹ¾ĒāŃõ»ÆĀĮµÄČܽā | |
| C£® | MµćŹ±£¬ČÜŅŗÖŠ³ĮµķµÄÖŹĮæŠ”ÓŚ3.9 g | |
| D£® | Ō»ģŗĻČÜŅŗÖŠµÄNa2CO3ČÜŅŗµÄÅضČĪŖ1 mol•L-1 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | øĆČÜŅŗÖŠ×ī¶ą“ęŌŚ4ÖÖĄė×Ó | |
| B£® | øĆČÜŅŗæÉÓÉKCl”¢£ØNH4£©2SO4ŗĶFeCl3ÅäÖʶų³É | |
| C£® | øĆČÜŅŗÖŠŅ»¶Ø“ęŌŚCl-”¢ĒŅc£ØCl-£©”Ż0.4mol•L-1 | |
| D£® | øĆČÜŅŗÖŠ²»ÄÜČ·¶ØŹĒ·ń“ęŌŚCO32- |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| Ń”Ļī | ŹŌ¼Į | ŹŌÖ½/ŹŌŅŗ | ĻÖĻó | ½įĀŪ |
| A | ÅØ°±Ė®”¢ÉśŹÆ»Ņ | ŗģÉ«ŹÆČļŹŌÖ½ | ±äĄ¶ | NH3ĪŖ¼īŠŌĘųĢå |
| B | ÅØŃĪĖį”¢ÅØĮņĖį | ×ĻÉ«ŹÆČļŹŌÖ½ | ±äŗģ | HClĪŖĖįŠŌĘųĢå |
| C | ÅØŃĪĖį”¢¶žŃõ»ÆĆĢ | µķ·Ūµā»Æ¼ŲŹŌÖ½ | ±äĄ¶ | Cl2¾ßÓŠŃõ»ÆŠŌ |
| D | ŃĒĮņĖįÄĘ”¢ĮņĖį | Ę·ŗģŹŌŅŗ | ĶŹÉ« | SO2¾ßÓŠ»¹ŌŠŌ |
| A£® | A | B£® | B | C£® | C | D£® | D |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
 Ti”¢Fe”¢Cr”¢MnµČ¾łĪŖ¹ż¶ÉŌŖĖŲ£¬ŌŚÉś²śÉś»īÖŠĘš×Ų»æÉĢę“śµÄÖŲŅŖ×÷ÓĆ£¬¶ŌĘ䵄֏ŗĶ»ÆŗĻĪļµÄÓ¦ÓĆŃŠ¾æŹĒÄæĒ°æĘѧъ¾æµÄĒ°ŃŲÖ®Ņ»£®Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
Ti”¢Fe”¢Cr”¢MnµČ¾łĪŖ¹ż¶ÉŌŖĖŲ£¬ŌŚÉś²śÉś»īÖŠĘš×Ų»æÉĢę“śµÄÖŲŅŖ×÷ÓĆ£¬¶ŌĘ䵄֏ŗĶ»ÆŗĻĪļµÄÓ¦ÓĆŃŠ¾æŹĒÄæĒ°æĘѧъ¾æµÄĒ°ŃŲÖ®Ņ»£®Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com