
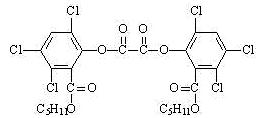
���յ�ҹ������������ͯ�ֳַ���ġ�ħ�����ڹ㳡����Ϸ�� ��ħ��������ԭ�������ù���������������������������������������ݸ�ӫ�����ʺ�㷢��ӫ�⣬�������(CPPO)�ṹ��ʽ��ͼ(ʽ������-C5H11��ͬ)������˵������ȷ���ǣ� ��
A��ħ��������̷�����������ԭ��Ӧ
B��1mol�����������������ϡ��Һ��Ӧʱ��
(������±�ز�ˮ��)���������6molNaOH
C���������ˮ����Եõ������л���
D��������ԭ�ӿ�����ͬһƽ��
 һ����ʦ�����Ծ�ϵ�д�
һ����ʦ�����Ծ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���ס��ҡ�����λͬѧ�ù涨��ҩƷ�Ʊ�Al(OH)3���涨���õ�ҩƷ���£�350 g 70% H2SO4��Һ��NaOH����240 g��������м��ˮ(����������ҩƷ)��
�ס��ҡ����ø�����Ƶķ����Ƶ�Al(OH)3�������ֱ���W1��W2��W3������ʵ�鷽�����£�
�ף�������NaOH��Һ����H2SO4��Һ��W1 g Al(OH)3
�ң�������H2SO4��Һ����NaOH��Һ��W2 g Al(OH)3
����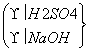 ��W3 g Al(OH)3
��W3 g Al(OH)3
�Իش�
(1)�ӳ������ԭ�ϡ��� �ͳɱ�����߲��ʵ����ط�����ʵ�鷽�����������________��
�ͳɱ�����߲��ʵ����ط�����ʵ�鷽�����������________��
(2)��λѧ���Ƶõ�Al(OH)3��W1��W2��W3�������ɴ�С��˳����________________________________________________________________________��
(3)�Ƶ�Al(OH)3�����������________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��������ABn������ԭ��A��û�й¶Ե��ӣ����ü۲���ӶԻ������ۣ�����˵����ȷ����(����)
A����n��2������ӵ�����ṹΪV��
B����n��3������ӵ�����ṹΪ������
C����n��4������ӵ�����ṹΪ����������
D������˵��������ȷ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij��ѧʵ��С����Ҫ�˽��г�������ʳ�ð״ף���Ҫ�Ǵ����ˮ��Һ����ȷŨ�ȣ��ִ��г�������һƿijƷ��ʳ�ð״ף���ʵ���ұ�NaOH��Һ������еζ���
 ��1����ʵ��Ӧѡ�� ��ָʾ����
��1����ʵ��Ӧѡ�� ��ָʾ����
��2����ͼ��ʾ50mL�ζ�����Һ���λ�ã���A��C�̶ȼ����1mL��A���� �̶�Ϊ25���ζ�����Һ�����ӦΪ mL��
��3��Ϊ�˼�Сʵ������ͬѧһ������������ʵ�飬����ÿ����ȡ�״������ΪVmL��NaOH��ҺŨ��Ϊc mo1/L������ʵ������¼���£�
| ʵ����� | ��һ�� | �ڶ��� | ������ |
| ����NaOH��Һ���/mL | 26.02 | 25.35 | 25.30 |
���ϱ����Կ�������һ��ʵ���м�¼����NaOH��Һ��������Զ��ں����Σ���ԭ������� ��
A��ʵ�����ʱ���ӿ̶��߶�ȡ�ζ��յ�ʱNaOH��Һ�����
B���ζ�ǰ�ζ��ܼ��������ݣ��ζ�����������
C��ʢװ��Һ�ĵζ���װҺǰ������ˮ��ϴ����δ�ñ�Һ��ϴ
D����һ�εζ��õ���ƿ�ô�װҺ��ϴ����������δ��ϴ��
E���μ�NaOH��Һ���죬δ������տ�����Һ��ɫ������ֹͣ�ζ�
��4�������������ݣ�д������ð״��д�������ʵ���Ũ�ȵı���ʽ�����ػ���
C��CH3COOH���� mo1/L��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ʹ9 g�Ҷ����ij��Ԫ����ȫ����������W g����3��6 gˮ����ô�����Է�������Ϊ�� ��
A��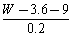 B��
B��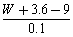 C��
C��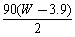 D��
D��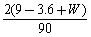
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij�л���A�ĺ�����ͺ˴Ź���������ͼ��ʾ,����˵���д�����ǣ� ��
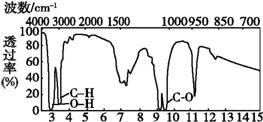
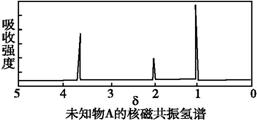
A.�ɺ������֪,���л��������������ֲ�ͬ�Ļ�ѧ��
B.�ɺ˴Ź�������֪,���л�������������ֲ�ͬ����ԭ��
C.��A�Ļ�ѧʽΪC2H6O������ṹ��ʽΪCH3-O-CH3
D.������˴Ź�����������֪������е���ԭ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����ж����и�ʴ�ԣ�ʹ��ʱ����������Ƥ���ϣ�������ϴ�ӵ��Լ���
A���ƾ� B��NaHCO3��Һ C��65�����ϵ�ˮ D����ˮ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����ӵĹ�ҵ��ˮ����������ͼ��ͼ��ʾ��
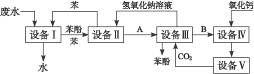
��1������������豸���н��е���_________��������д�������ƣ���ʵ��������һ������������__________________����д�������ƣ����С�
��2�����豸������豸�������A��_________�����豸������豸��������B��_________��
��3�����豸���з�����Ӧ�Ļ�ѧ����ʽΪ��_______________________________________��
��4�����豸���У�����B��ˮ��Һ��CaO��Ӧ������NaOH��H2O��_________��ͨ������________________����д�������ƣ�������ʹ��������롣
��5��ͼ�У���ѭ��ʹ�õ�������C6H6��CaO��________________��________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��FeCl3��CuCl2�����Һ�У�����һ��������м����Ӧ��ȫ�����˳�������˵������ȷ���� (����)
A�����˳��Ĺ�����ֻ��ͭ������Һ��һ�����е���������Fe2����һ������Cu2��
B�����˳��Ĺ�����ֻ��ͭ������Һ��һ�����е���������Fe2�������ܺ�Cu2����Fe3��
C�����˳��Ĺ�����ֻ��ͭ������Һ��һ�����е���������Fe3����Fe2����һ������Cu2��
D�����˳��Ĺ����к�������ͭ������Һ��һ�����е���������Fe2����һ������Cu2����Fe3��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com