
¼×ŅŅĮ½Ī»Ķ¬Ń§·Ö±šÓĆ²»Ķ¬µÄ·½·ØÅäÖĘ100 mL 3.6 mol/LµÄĻ”ĮņĖį”£
(1)Čō²ÉÓĆ18 mol/LµÄÅØĮņĖįÅäÖĘČÜŅŗ,ŠčŅŖÓƵ½ÅØĮņĖįµÄĢå»żĪŖ”””””””””””””””””£
(2)¼×ѧɜ:ĮæČ”ÅØĮņĖį,Š”ŠÄµŲµ¹ČėŹ¢ÓŠÉŁĮæĖ®µÄÉÕ±ÖŠ,½Į°č¾łŌČ,“żĄäČ“ÖĮŹŅĪĀŗó×ŖŅʵ½100 mLČŻĮæĘæÖŠ,ÓĆÉŁĮæµÄĖ®½«ÉÕ±µČŅĒĘ÷Ļ“µÓ2”«3“Ī,Ćæ“ĪĻ“µÓŅŗŅ²×ŖŅʵ½ČŻĮæĘæÖŠ,Č»ŗ󊔊ĵŲĻņČŻĮæĘæ¼ÓČėĖ®ÖĮæĢ¶ČĻ߶ØČŻ,ČūŗĆĘæČū,·“ø“ÉĻĻĀµßµ¹Ņ”ŌČ”£
¢Ł½«ČÜŅŗ×ŖŅʵ½ČŻĮæĘæÖŠµÄÕżČ·²Ł×÷ŹĒ
Čō¶ØČŻŹ±ŃöŹÓæĢ¶ČĻß,ŌņĖłÅäČÜŅŗÅØ¶Č””””””””(Ģī”°Ę«“ó”±”¢”°Ę«Š””±»ņ”°ĪŽÓ°Ļģ”±)”£
¢ŚĻ“µÓ²Ł×÷ÖŠ,½«Ļ“µÓÉÕ±ŗóµÄĻ“ŅŗŅ²×¢ČėČŻĮæĘæ,ĘäÄæµÄŹĒ”” ”£
¢Ū¶ØČŻµÄÕżČ·²Ł×÷ŹĒ”” ”£
¢ÜÓĆ½ŗĶ·µĪ¹ÜĶłČŻĮæĘæÖŠ¼ÓĖ®Ź±,²»Š”ŠÄŅŗĆę³¬¹żĮĖæĢ¶Č,“¦ĄķµÄ·½·ØŹĒ””””””””(ĢīŠņŗÅ)”£
A.Īü³ö¶ąÓąŅŗĢå,Ź¹°¼ŅŗĆęÓėæĢ¶ČĻßĻąĒŠ
BŠ”ŠÄ¼ÓČČČŻĮæĘæ,¾Õō·¢ŗó,Ź¹°¼ŅŗĆęÓėæĢ¶ČĻßĻąĒŠ
C.¾¼ĘĖć¼ÓČėŅ»¶ØĮæµÄÅØŃĪĖį
D.ÖŲŠĀÅäÖĘ
(3)ŅŅѧɜ:ÓĆ100 mLĮæĶ²ĮæČ”ÅØĮņĖį,²¢ĻņĘäÖŠŠ”ŠÄµŲ¼ÓČėÉŁĮæĖ®,½Į°č¾łŌČ,“żĄäČ“ÖĮŹŅĪĀŗó,ŌŁ¼ÓČėĖ®ÖĮ100 mLæĢ¶ČĻß,ŌŁ½Į°č¾łŌČ”£ÄćČĻĪŖ“Ė·ØŹĒ·ńÕżČ·?Čō²»ÕżČ·,Öø³öĘäÖŠ“ķĪóÖ®“¦:”” ”£

| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
(8·Ö)(1) 0£®5 mol SO2¹²Ō¼ŗ¬ÓŠ øöŌ×Ó£¬ĖüÓė gSO3Ėłŗ¬ĮņŌ×ÓŹżĻąµČ”£
£Ø2£©ÖŹĮæĻąĶ¬µÄ ¢ŁHCl”¢¢ŚNH3”¢¢ŪCO2”¢¢ÜO2ĖÄÖÖĘųĢåÖŠ£¬ŗ¬ÓŠ·Ö×ÓŹżÄæ×īÉŁµÄŹĒ(ĢīŠņŗÅ) _ ”£
£Ø3£©ÓŠ100mL 0£®2 mol/L µÄNaOHČÜŅŗ£¬½«“ĖČÜŅŗĻ”ŹĶµ½200 mL£¬ŌņČÜŅŗÖŠNa+µÄĪļÖŹµÄĮæŹĒ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ŌŚ120”ę”¢101 kPaĢõ¼žĻĀ£¬ÓÉH2”¢CH4”¢CO×é³ÉµÄ»ģŗĻĘųĢåa mL£¬ĶØČėŅ»¶ØĮæ(ÉčĪŖx mL)ŃõĘųŹ¹ĘäĶźČ«Č¼ÉÕ”£
(1)Čōa mL»ģŗĻĘųĢåĶźČ«Č¼ÉÕĻūŗÄĻąĶ¬Ģõ¼žĻĀŃõĘųµÄĢå»żŅ²ĪŖa mL£¬(¼“x£½a)£¬ŌņŌ»ģŗĻĘųĢåÖŠCH4µÄĢå»ż·ÖŹżŹĒ £»
(2)ČōĶźČ«Č¼ÉÕŗóÉś³ÉCO2ŗĶH2O(g)µÄ×ÜĢå»żŌŚĻąĶ¬Ģõ¼žĻĀĪŖ2a mL£¬ŌņŌ»ģŗĻĘųĢåÖŠCH4µÄĢå»ż·ÖŹżŹĒ £¬ĻÖŅŖ²ā¶ØŌ»ģŗĻĘųĢåÖŠH2µÄĢå»ż·ÖŹż£¬»¹±ŲŠėÖŖµĄĻąĶ¬Ģõ¼žĻĀµÄĘäĖūŹż¾Ż£¬æÉŅŌŹĒ (ĢīŃ”Ļī×ÖÄø)£»
A£®2a mL»ģŗĻĘųĢåµÄĆܶČ
B£®Éś³ÉCO2ĘųĢåµÄ×ÜĢå»ż
C£®Éś³ÉH2O(g)µÄ×ÜÖŹĮæ
(3)ČōŌ»ģŗĻĘųĢåĶźČ«Č¼ÉÕŹ±£¬Éś³ÉµÄĘųĢåÖŠÖ»ÓŠCO2ŗĶH2O(g)£¬ŌņxµÄȔֵ·¶Ī§ŹĒ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
£Ø1£©ĻĀĮŠĪļÖŹÖŠĖ®ČÜŅŗÄܵ¼µēµ«ŹōÓŚ·Ēµē½āÖŹµÄÓŠ £ØĢīŠņŗÅ£©¢ŁŅŅ“¼ ¢Ś°±Ęų ¢ŪĀČĘų ¢ÜĀČ»ÆÄĘ ¢ŻĮņĖį ¢Žøɱł ¢ßBaSO4 ¢ą“×Ėį ¢įĒāŃõ»ÆÄĘ ¢āCaO
£Ø2£©Ä³ŹµŃéŠčŅŖŹ¹ÓĆ240ml 0.4mol/L CuSO4ČÜŅŗ£¬ÓĆµØ·ÆÅäÖĘøĆÅضČČÜŅŗŠčŅŖŹ¹ÓƵÄŅĒĘ÷ÓŠĶŠÅĢĢģĘ½”¢ÉÕ±”¢²£Į§°ō”¢ ”¢ £»ŠčŅŖ³ĘĮæ æĖµØ·Æ£»ČōĖł³ĘµØ·ÆŹ§Č„²æ·Ö½į¾§Ė®£¬ŌņÅäÖĘ³öµÄČÜŅŗÅØ¶Č ”££ØĢīĘ«øß”¢Ę«µĶ»ņĪŽÓ°Ļģ£©
£Ø3£©ŗŗ×ĻŹĒÖŠ¹ś¹Å“ś±ųĀķŁøŗĶ¹Å“ś±Ś»ÖŠµÄŅ»ÖÖŃÕĮĻ£¬Ęä»ÆѧŹ½ŹĒBaCuSi2O6£¬ĒėÓĆŃõ»ÆĪļµÄŠĪŹ½±ķŹ¾Ęä×é³É£ŗ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ŗĻ³É°±¹¤ŅµÉś²śÖŠĖłÓƵĦĮFe“߻ƼĮµÄÖ÷ŅŖ³É·ÖŹĒFeO”¢Fe2O3”£
£Ø1£©Ä³FeO”¢Fe2O3»ģŗĻĪļÖŠ£¬Ģś”¢ŃõµÄĪļÖŹµÄĮæÖ®±ČĪŖ4”Ć5£¬ĘäÖŠFe2£«ÓėFe3£«ĪļÖŹµÄĮæÖ®±ČĪŖ________”£
£Ø2£©µ±“߻ƼĮÖŠFe2£«ÓėFe3£«µÄĪļÖŹµÄĮæÖ®±ČĪŖ1”Ć2Ź±£¬Ęä“߻ƻīŠŌ×īøߣ¬“ĖŹ±ĢśµÄŃõ»ÆĪļ»ģŗĻĪļÖŠĢśµÄÖŹĮæ·ÖŹżĪŖ________£ØÓĆŠ”Źż±ķŹ¾£¬±£Įō2Ī»Š”Źż£©”£
£Ø3£©ŅŌFe2O3ĪŖŌĮĻÖʱøÉĻŹö“߻ƼĮ£¬æÉĻņĘäÖŠ¼ÓČėŹŹĮæĢ¼·Ū£¬·¢ÉśČēĻĀ·“Ó¦£ŗ2Fe2O3£«C 4FeO£«CO2”ü”£
4FeO£«CO2”ü”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
µŖŹĒµŲĒņÉĻŗ¬Įæ·įø»µÄŅ»ÖÖŌŖĖŲ£¬°±”¢ėĀ(N2H4)ŗĶµžµŖĖį¶¼ŹĒµŖŌŖĖŲµÄÖŲŅŖĒā»ÆĪļ£¬ŌŚ¹¤Å©ŅµÉś²ś”¢Éś»īÖŠÓŠ×ÅÖŲ“ó×÷ÓĆ”£
(1)ŗĻ³É°±Éś²ś¼¼ŹõµÄ““Į¢æŖ±ŁĮĖČĖ¹¤¹ĢµŖµÄĶ¾¾¶£¬¶Ō»Æѧ¹¤Ņµ¼¼ŹõŅ²²śÉśĮĖÖŲŅŖÓ°Ļģ”£
¢ŁŌŚ¹Ģ¶ØĢå»żµÄĆܱÕČŻĘ÷ÖŠ£¬½ųŠŠČēĻĀ»Æѧ·“Ó¦£ŗN2(g)£«3H2(g)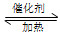 2NH3(g)””¦¤H£¼0£¬ĘäĘ½ŗā³£ŹżKÓėĪĀ¶ČTµÄ¹ŲĻµČēĻĀ±ķ”£
2NH3(g)””¦¤H£¼0£¬ĘäĘ½ŗā³£ŹżKÓėĪĀ¶ČTµÄ¹ŲĻµČēĻĀ±ķ”£
| T/K | 298 | 398 | 498 |
| Ę½ŗā³£ŹżK | 4.1”Į106 | K1 | K2 |
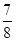 £¬ŌņN2µÄ×Ŗ»ÆĀŹĪŖ________£¬ŅŌNH3µÄÅØ¶Č±ä»Æ±ķŹ¾øĆ¹ż³ĢµÄ·“Ó¦ĖŁĀŹĪŖ________”£
£¬ŌņN2µÄ×Ŗ»ÆĀŹĪŖ________£¬ŅŌNH3µÄÅØ¶Č±ä»Æ±ķŹ¾øĆ¹ż³ĢµÄ·“Ó¦ĖŁĀŹĪŖ________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
°“ŅŖĒóĶź³ÉĻĀĮŠĪŹĢā£ŗ
£Ø1£©0.5 mol H2OµÄÖŹĮæĪŖ g£¬¹²ÓŠ____________øö·Ö×Ó”£
£Ø2£© 0.01molijĪļÖŹµÄÖŹĮæĪŖ1.08g£¬Ōņ“ĖĪļÖŹµÄĦ¶ūÖŹĮæĪŖ__________________”£
£Ø3£©ÅäÖĘ50 mL 0.2 mol”¤L”Ŗ1 CuSO4ČÜŅŗ£¬ŠčŅŖCuSO4_____________g”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ĻÖÓŠm gijĘųĢ壬ĖüÓÉĖÄŌ×Ó·Ö×Ó¹¹³É£¬ĖüµÄĦ¶ūÖŹĮæĪŖM g”¤mol£1”£Ōņ£ŗ
£Ø1£©øĆĘųĢåµÄĪļÖŹµÄĮæĪŖ____ ____”£
£Ø2£©øĆĘųĢåÖŠĖłŗ¬µÄŌ×Ó×ÜŹżĪŖ________øö”£
£Ø3£©øĆĘųĢåŌŚ±ź×¼×“æöĻĀµÄĢå»żĪŖ________L”£
£Ø4£©øĆĘųĢåČÜÓŚ1 LĖ®ÖŠ(²»æ¼ĀĒ·“Ó¦)£¬ĘäČÜŅŗÖŠČÜÖŹµÄÖŹĮæ·ÖŹżĪŖ________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
½«ŗ¬ÓŠ2.05 gijøß¼Ūŗ¬ŃõĖįµÄøĘŃĪµÄČÜŅŗAÓėŗ¬1.20 gĢ¼ĖįŃĪµÄČÜŅŗB»ģŗĻ£¬Ē”ŗĆĶźČ«·“Ó¦£¬Éś³É1.25 g°×É«³ĮµķC”£½«ĀĖČ„³ĮµķCµÄĀĖŅŗÕō·¢£¬µĆµ½°×É«¹ĢĢåD£¬¼ĢŠų¼ÓČČDŹ±£¬D·Ö½āÖ»µĆĮ½ÖÖĘųĢ¬ĪļÖŹµÄ»ģŗĻĪļ£¬ŌŚ0 ”ę”¢1”Į105 PaĻĀ£¬Ģå»ż±äĪŖ0.56 L£¬²¢µĆµ½0.90 gŅŗĢ¬Ė®£¬ĮķŅ»ÖÖĘųĢ¬ĪļÖŹĪŖĘųĢ¬Ńõ»ÆĪļR2O”£ŹŌ»Ų“š£ŗ
(1)°×É«³ĮµķCµÄĪļÖŹµÄĮæĪŖ________mol”£
(2)AµÄĦ¶ūÖŹĮæĪŖ__________£¬BµÄĦ¶ūÖŹĮæĪŖ__________”£
(3)R2OÓėH2OµÄĪļÖŹµÄĮæÖ®±ČĪŖ__________£¬Éś³ÉDµÄÖŹĮæĪŖ________£¬DµÄĦ¶ūÖŹĮæĪŖ________£¬R2OµÄĻą¶Ō·Ö×ÓÖŹĮæĪŖ________£¬R2OµÄ»ÆѧŹ½ŹĒ____________”£
(4)Š“³öAŗĶB»ģŗĻµÄ»Æѧ·½³ĢŹ½_____________________________________________”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com