
һ���ѧ�о��ɹ�������ͭ��������(CuMn2O4)���ڳ����´����������е�һ����̼�ͼ�ȩ(HCHO)��
(1)��һ�����ʵ���Ũ�ȵ�Cu(NO3)2��Mn(NO3)2��Һ�м���Na2CO3��Һ�����ó������������գ����Ƶ�CuMn2O4��
��Mn2����̬�ĵ����Ų�ʽ�ɱ�ʾΪ________��
��NO3���Ŀռ乹����________(����������)��
(2)��ͭ��������Ĵ��£�CO������ΪCO2��HCHO������ΪCO2��H2O��
�ٸ��ݵȵ�����ԭ����CO���ӵĽṹʽΪ________��
��H2O������Oԭ�ӹ�����ӻ�����Ϊ________��
��1 mol CO2�к��еĦҼ���ĿΪ________��
(3)��CuSO4��Һ�м������NaOH��Һ������[Cu(OH)4]2���������ǿռ乹�ͣ�[Cu(OH)4]2���Ľṹ����ʾ��ͼ��ʾΪ_______________________________
 ���ٴ�����ɽ����ϵ�д�
���ٴ�����ɽ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
Ϊ����·���ѩ����ʹ����һ����ѩ��������Ҫ�ɷֵĻ�ѧʽΪXY2��X��Y��Ϊ���ڱ�ǰ20��Ԫ�أ��������Ӻ������ӵĵ��Ӳ�ṹ��ͬ����1 mol XY2����54 mol���ӡ�
��1������ѩ���Ļ�ѧʽ��________���������л�ѧ��������________������ʽ��________��
��2��Ԫ��D��Eԭ�ӵ�����������������Ӳ�����2����D��Y���ڣ���D�����ӽṹʾ��ͼ��________��D��E���γ�һ�ֽṹ������CO2����ԭ�ӷ��ӣ���ÿ��ԭ�Ӿ��ﵽ��8e���ȶ��ṹ���÷��ӵĽṹʽΪ________������ʽΪ________����ѧ������Ϊ________������Ӽ������Ǽ��Թ��ۼ������Թ��ۼ�������
��3��W����Dͬ����Ķ�����Ԫ�أ�Z�ǵ������ڽ�������ǿ��Ԫ�أ�Z�ĵ�����W�ij��������з�Ӧʱ�����ֲ��������ʱ����______���仯ѧ������Ϊ________������ʱ����______���仯ѧ������Ϊ________������ʽΪ________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
A��B��C��D��E�����ڱ���ǰ�����ڵ�Ԫ��,���й����ʻ�ṹ��Ϣ���±�:
| Ԫ�� | �й����ʻ�ṹ��Ϣ |
| A | �����۵�AԪ�ص��⻯����ͨ��״������һ��Һ��,����A����������Ϊ88.9% |
| B | Bԭ�ӵõ�һ�����Ӻ�3p���ȫ���� |
| C | Cԭ�ӵ�p��������,������̬�⻯������������������ˮ���ﷴӦ����һ�ֳ�������X |
| D | DԪ�ص�����ϼ�����ͻ��ϼ۵Ĵ�����Ϊ��,�����������Ϊ���Ӿ��� |
| E | EԪ�صĺ˵��������AԪ�غ�BԪ���⻯��ĺ˵����֮�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
A��B��C��D��E���ֶ�����Ԫ�ص�ԭ��������������Ԫ��A�ĸ�һ�����Ӿ��к�ϡ������Heһ���Ľṹ��Ԫ��B���������������ڲ����������2����Ԫ��C�Ƕ�����Ԫ����ԭ�Ӱ뾶��������Ԫ�أ�Ԫ��D�ڵؿ��еĺ���λ�ڵڶ�λ��Ԫ��E��Ԫ��B���γɾ�����������ṹ�����ʡ���ش��������⣺
��1�������ڱ��У�Ԫ��Bλ�ڵ� ���ڵ� �壻A��C�γɵ����ӻ�����Ļ�ѧʽΪ _________________ �� A��C�γɵ����ӻ�������ˮ��Ӧ�Ļ�ѧ����ʽΪ ��
��2��C�γɵļ����ӵ����ӽṹʾ��ͼΪ ��
��3��D��EԪ���γɵĻ��������ˮ��Ӧ����һ�ֳ�����һ�����壬��д���÷�Ӧ�Ļ�ѧ����ʽ�� ___________ ��
��4������B��ˮ��Ӧ�ǽ�B������õĴ�ʩ֮һ����д���÷�Ӧ�Ļ�ѧ����ʽ�� ______________________________________ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ͼ�Ǵ�Ԫ�����ڱ��н�ȡ��һ��Ƭ�ϣ�����X��Y��Z��W�����ڶ�����Ԫ�ء���ش��������⡣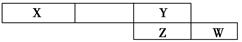
(1)��X����Ϊ�����е���Ҫ�ɷ�֮һ����W��ԭ�ӽṹʾ��ͼΪ________��
(2)��Y��W��Z������������Ӧ��ˮ��������ΪһԪ�ᡢ��Ԫ�ᡢ��Ԫ�ᣬ������������εĺ�����������У������ֵĵ�������ȣ����������ӵ����ӷ�����________��________��
(3)��Na��Y��Z�ĵ��ʷֱ�Ӧ��������Ħ��������ͬ�����ֻ��������Na��Y�γɵĻ�����ĵ���ʽΪ________��������ѧ��������Ϊ________��
(4)YԪ����Ԫ�����ڱ��д��ڵ�________����(д�����п���������)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
A��B��C��D��E������ԭ���������ε����Ķ�����Ԫ�أ���֪������ֻ��һ���ǽ���Ԫ�أ�A��DԪ�ص�ԭ��������������ͬ��C��Eͬ���壬��EԪ��ԭ����������CԪ��ԭ����������2����B�������������ǵ��Ӳ�����������C��A���γ����ֳ�����Һ̬��������ң�����Է��������ұȼ״�16��
��1��EԪ�ص�����Ϊ�� ��D��ԭ�ӽṹʾ��ͼΪ ��
��2�����������к��еĻ�ѧ���� ������Թ��ۼ����Ǽ��Թ��ۼ�������
��3��A������B��C�γɵĻ�����ɺϳ�һ��������������ȼ�ϼ״���
��֪CO��g��+1/2O2��g��=CO2��g��
��H=��283 kJ/mol
H2��g��+1/2O2��g��===H2O��g�� ��H=��242 kJ/mol
CH3OH��g��+3/2O2��g��=CO2��g��+2H2O��g��
��H=��651 kJ/mol
д��A����ѡ������ϳɼ״����Ȼ�ѧ����ʽ�� ��
��4��EC2��C��D�γɵĻ������������ԭ��Ӧ�Ļ�ѧ����ʽΪ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������Ԫ��A��X��D��E��R��Tԭ��������������ԭ�ӽṹ�����������ʾ��
| Ԫ�� | �ṹ������ |
| A | A��ԭ�Ӱ뾶��С |
| X | Xԭ�������������Ǵ��������� |
| D | D�Ƕ������н�������ǿ��Ԫ�� |
| E | E������������Ӧˮ������һ�ֳ����������������� |
| R | R��Xͬ���� |
| T | T�ĸ�һ�������ӵĺ�������Ų���Arԭ����ͬ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��14�֣�A��B��C��D��E��F��Ϊ������Ԫ�أ�ԭ���������ε�����AԪ��ԭ�Ӻ��������ӡ�BԪ��ԭ�������������Ǵ�����������2����CԪ���ǵؿ��Ǻ�������Ԫ�ء�D�Ƕ�����Ԫ���н�������ǿ��Ԫ�ء�E��F��λ�����ڣ�F������������ˮ����Ϊ��ǿ���ᡣ
��1���ƶ�B��Ԫ�����ڱ��е�λ�ã� ��
��2��д��A��C�γɵ�10���ӵ������ӻ�ѧʽ�� �����ö�Ӧ�Ļ�ѧ������գ���ͬ��
��3��E��F����Ԫ���зǽ����Խ�ǿ���� ���õ���ʽ��ʾD2C�γɹ���
��4��D��E�γɵ����ε�ˮ��Һ�У������ӵ�Ũ�ȴ�С˳��Ϊ�� ���Ӵ�С���У���
��5����������1molAԪ�صĵ�����CԪ�صĵ��ʻ��ϣ��ų�286kJ��������д����Ӧ���Ȼ�ѧ����ʽ��
��6������A��C��ԭ�Ӹ�����1:1��ɵĻ������֪����Һ��ʹ���Ը��������Һ��ɫ��������0.5mol����Һ�μӵ�100mL 2mol/L���Ը��������Һ�У���Һ��ɫǡ����ȥ���÷�Ӧ�����ӷ���ʽΪ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
A��B��C��D�����ֶ�����Ԫ�أ����ǵ�ԭ������������������AԪ�ص�ԭ��ֻ��һ�����Ӳ㣬��֪A��C��B��D�ֱ�����ͬһ����Ԫ�أ�B��D��Ԫ�ص�ԭ�Ӻ���������֮����A��C��Ԫ��ԭ�Ӻ���������֮�͵Ķ�������֪����Ԫ�صĵ��ʳ��³�ѹ�����������塢���ֹ��塣��ش��������⣺
��1��DԪ����Ԫ�����ڱ��е�λ�ã� ��
��2��BԪ�ر�DԪ�صķǽ�����ǿ�������� ������ţ���
a����Ԫ����ɵĻ�������DԪ��Ϊ���� b�����ʵ��۷е�ĸߵ�
c������������Ӧ��ˮ���������ǿ�� d����̬�⻯����ȶ���
��3����A��B��D����Ԫ���е����ֿɷֱ��γɼס����������ӣ����Ǿ�Ϊ��һ��˫ԭ�Ӻ˵������ӣ��Ҽ����Ӻ���18�����ӣ������Ӻ���10�����ӣ�������ҷ�Ӧ�����ӷ���ʽΪ ��
��4��д��C��D��Ԫ�ص�����������Ӧ��ˮ������ϡ��Һ�ﷴӦ���Ȼ�ѧ����ʽ�� ����֪�˷�Ӧ����1 molH2Oʱ�ų�������Ϊ57.3 kJ����
��5����AԪ�صĵ�����BԪ�صĵ��ʿ����Ƴ�ȼ�ϵ�أ�ȼ�ϵ����װ��KOHŨ��Һ���ö�Ľ������Ե缫����KOH��Һ����M��ͨ��A�ĵ��ʣ�N��ͨ��B�ĵ��ʣ���N���ĵ缫��ӦʽΪ�� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com