
“æĶŌŚ¹¤ŅµÉĻÖ÷ŅŖÓĆĄ“ÖĘŌģµ¼Ļß”¢µēĘ÷ŌŖ¼žµČ£¬ĶÄÜŠĪ³É£«1¼ŪŗĶ£«2¼ŪµÄ»ÆŗĻĪļ”£
(1)Š“³ö»łĢ¬Cu£«µÄŗĖĶāµē×ÓÅŲ¼Ź½___________________________________”£
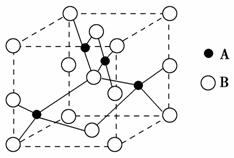 (2)ČēĶ¼ŹĒĶµÄijÖÖŃõ»ÆĪļµÄ¾§°ūŹ¾ŅāĶ¼£¬øĆŃõ»ÆĪļµÄ»ÆѧŹ½ĪŖ________”£
(2)ČēĶ¼ŹĒĶµÄijÖÖŃõ»ÆĪļµÄ¾§°ūŹ¾ŅāĶ¼£¬øĆŃõ»ÆĪļµÄ»ÆѧŹ½ĪŖ________”£
(3)ĻņĮņĖįĶČÜŅŗÖŠµĪ¼Ó°±Ė®»įÉś³ÉĄ¶É«³Įµķ£¬ŌŁµĪ¼Ó°±Ė®µ½³ĮµķøÕŗĆČ«²æČܽāæɵƵ½ÉīĄ¶É«ČÜŅŗ£¬¼ĢŠųĻņĘäÖŠ¼ÓČė¼«ŠŌ½ĻŠ”µÄŅŅ“¼æÉŅŌÉś³ÉÉīĄ¶É«µÄ[Cu(NH3)4]SO4”¤H2O³Įµķ£¬øĆĪļÖŹÖŠµÄNH3Ķعż________¼üÓėÖŠŠÄĄė×ÓCu2£«½įŗĻ£¬NH3·Ö×ÓÖŠNŌ×ÓµÄŌӻƷ½Ź½ŹĒ____”£ÓėNH3·Ö×Ó»„ĪŖµČµē×ÓĢåµÄŅ»ÖÖĪ¢Į£ŹĒ________”£
(4)CuOµÄČŪµć±ČCuClµÄČŪµć____(Ģī”°øß”±»ņ”°µĶ”±)”£
½āĪö””(1)Cu¼Ūµē×Ó¹¹ŠĶŹĒ3d104s1£¬Ź§µē×ÓŹ±ĻČŹ§Č„×īĶā²ćµÄ1øöµē×Ó£¬¹Ź»łĢ¬Cu£«µÄµē×ÓÅŲ¼Ź½ĪŖ1s22s22p63s23p63d10”£
(2)ĶĄė×Ó°ė¾¶“óÓŚŃõĄė×Ó°ė¾¶£¬¹ŹAĪŖŃõĄė×Ó£¬BĪŖĶĄė×Ó£¬¾§°ūÖŠCuĄė×ÓÓŠ8øöŌŚ¶„½Ē£¬4øöŌŚĄā±ßÉĻ£¬ÓŠ2øö·Ö±š“¦ÓŚÉĻ”¢ĻĀĆęŠÄÉĻ£¬ÓŠ1øöŌŚĢåŠÄ£¬Ōņ1øö¾§°ūÖŠĶĄė×ÓŹżĪŖ£ŗ1£«2”Į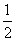 £«4”Į
£«4”Į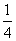 £«8”Į
£«8”Į £½4£¬OĄė×ÓČ«²æŌŚ¾§°ūÄŚ²æ£¬1øö¾§°ūÖŠŗ¬ÓŠ4øö£¬¹ŹĘä»ÆѧŹ½ĪŖCuO”£
£½4£¬OĄė×ÓČ«²æŌŚ¾§°ūÄŚ²æ£¬1øö¾§°ūÖŠŗ¬ÓŠ4øö£¬¹ŹĘä»ÆѧŹ½ĪŖCuO”£
(3)Cu2£«ÓėĶā½ēµÄSO42£ŅŌĄė×Ó¼üĻą½įŗĻ£¬ÓėNH3ŅŌÅäĪ»¼üĻą½įŗĻ£»NH3ÖŠNŌ×ÓµÄŌӻƷ½Ź½ŹĒsp3Ōӻƣ»ÓėNH3·Ö×Ó»„ĪŖµČµē×ÓĢåµÄĪ¢Į£ÓŠH3O£«”¢PH3”¢CH3£µČ”£
(4)CuOÖŠŃõĄė×Ó°ė¾¶Š”ÓŚCuClÖŠCl£°ė¾¶£¬Cu2£«°ė¾¶Š”ÓŚCu£«°ė¾¶£¬¹ŹCuOµÄ¾§øńÄÜ“óÓŚCuClµÄ¾§øńÄÜ£¬ŌņCuOµÄČŪµć±ČCuClµÄČŪµćøß”£
“š°ø””(1)1s22s22p63s23p63d10»ņ[Ar]3d10
(2)CuO
(3)ÅäĪ»””sp3””H3O£«»ņPH3µČ
(4)øß


 æŚĖ抔דŌŖæŚĖćĖŁĖćĢģĢģĮ·ĻµĮŠ“š°ø
æŚĖ抔דŌŖæŚĖćĖŁĖćĢģĢģĮ·ĻµĮŠ“š°ø ĢģĢģĮ·æŚĖćĻµĮŠ“š°ø
ĢģĢģĮ·æŚĖćĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĮŠĪļÖŹÖŠ¼ČŹōÓŚ·¼Ļć×å»ÆŗĻĪļÓÖŹōÓŚ“¼µÄŹĒ£Ø””””£©
| ”” | A£® |
| B£® |
| C£® |
| D£® | CH3CH2OH |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĒąĘ»¹ūÖÓöµāČÜŅŗĻŌĄ¶É«£¬ŹģĘ»¹ūÄÜ»¹ŌŅų°±ČÜŅŗ”£ÕāĖµĆ÷(””””)
A£®ĒąĘ»¹ūÖŠÖ»ŗ¬µķ·Ū²»ŗ¬ĢĒĄą
B£®ŹģĘ»¹ūÖŠÖ»ŗ¬ĢĒĄą²»ŗ¬µķ·Ū
C£®Ę»¹ū³ÉŹģŹ±µķ·ŪĖ®½āĪŖµ„ĢĒ
D£®Ę»¹ū³ÉŹģŹ±µ„ĢĒ¾ŪŗĻ³Éµķ·Ū
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ijŹµŃ銔×éÓĆĻĀĮŠ×°ÖĆ½ųŠŠŅŅ“¼“ß»ÆŃõ»ÆµÄŹµŃ锣

Ķ¼CB34
(1)ŹµŃé¹ż³ĢÖŠĶĶų³öĻÖŗģÉ«ŗĶŗŚÉ«½»ĢęµÄĻÖĻó£¬ĒėŠ“³öĻąÓ¦µÄ»Æѧ·½³ĢŹ½£ŗ________________________________________________________________________£»
ŌŚ²»¶Ļ¹ÄČėæÕĘųµÄĒéæöĻĀ£¬ĻØĆš¾Ę¾«µĘ£¬·“Ó¦ČŌÄܼĢŠų½ųŠŠ£¬ĖµĆ÷ŅŅ“¼µÄŃõ»Æ·“Ó¦ŹĒ________·“Ó¦”£
(2)¼×ŗĶŅŅĮ½øöĖ®Ō”×÷ÓĆ²»ĻąĶ¬”£¼×µÄ×÷ÓĆŹĒ________£»ŅŅµÄ×÷ÓĆŹĒ________”£
(3)·“Ó¦½ųŠŠŅ»¶ĪŹ±¼äŗó£¬ŹŌ¹ÜaÖŠÄÜŹÕ¼Æµ½²»Ķ¬µÄĪļÖŹ£¬ĖüĆĒŹĒ________________£»¼ÆĘųĘæÖŠŹÕ¼Æµ½µÄĘųĢåµÄÖ÷ŅŖ³É·ÖŹĒ________”£
(4)ČōŹŌ¹ÜaÖŠŹÕ¼Æµ½µÄŅŗĢåÓĆ×ĻÉ«ŹÆČļŹŌÖ½¼ģŃ飬ŹŌÖ½ĻŌŗģÉ«£¬ĖµĆ÷ŅŗĢåÖŠ»¹ŗ¬ÓŠ________”£ŅŖ³żČ„øĆĪļÖŹ£¬æÉĻČŌŚ»ģŗĻŅŗÖŠ¼ÓČė________(ĢīŠ“×ÖÄø)£¬Č»ŗóŌŁĶعż________(ĢīŹµŃé²Ł×÷Ćū³Ę)¼“æɵƵ½²śĪļ”£
a£®ĀČ»ÆÄĘČÜŅŗ””””b£®±½””””c£®Ģ¼ĖįĒāÄĘČÜŅŗ””””d£®ĖÄĀČ»ÆĢ¼
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
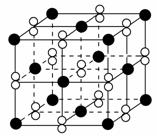 øßĪĀĻĀ£¬³¬Ńõ»Æ¼Ų¾§Ģå(KO2)³ŹĮ¢·½Ģå½į¹¹”£ČēĶ¼ĪŖ³¬Ńõ»Æ¼Ų¾§ĢåµÄŅ»øö¾§°ū(¾§ĢåÖŠ×īŠ”µÄÖŲø“µ„ŌŖ)”£ŌņĻĀĮŠÓŠ¹ŲĖµ·ØÕżČ·µÄŹĒ (””””)”£
øßĪĀĻĀ£¬³¬Ńõ»Æ¼Ų¾§Ģå(KO2)³ŹĮ¢·½Ģå½į¹¹”£ČēĶ¼ĪŖ³¬Ńõ»Æ¼Ų¾§ĢåµÄŅ»øö¾§°ū(¾§ĢåÖŠ×īŠ”µÄÖŲø“µ„ŌŖ)”£ŌņĻĀĮŠÓŠ¹ŲĖµ·ØÕżČ·µÄŹĒ (””””)”£
A£®KO2ÖŠÖ»“ęŌŚĄė×Ó¼ü
B£®³¬Ńõ»Æ¼ŲµÄ»ÆѧŹ½ĪŖKO2£¬Ćæøö¾§°ūŗ¬ÓŠ1øöK£«ŗĶ1øöO2£
C£®¾§ĢåÖŠÓėĆæøöK£«¾ąĄė×ī½üµÄO2£ÓŠ6øö
D£®¾§ĢåÖŠ£¬ĖłÓŠŌ×ÓÖ®¼ä¶¼ŅŌĄė×Ó¼üĻą½įŗĻ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
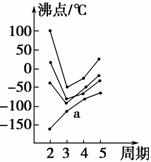 ČēĶ¼ÖŠĆæĢõÕŪĻß±ķŹ¾ÖÜĘŚ±ķ¢ōA×唫¢÷A×åÖŠµÄijŅ»×åŌŖĖŲĒā»ÆĪļµÄ·Šµć±ä»Æ£¬ĆæøöŠ”ŗŚµć“ś±ķŅ»ÖÖĒā»ÆĪļ£¬ĘäÖŠaµć“ś±ķµÄŹĒ £Ø””””£©”£
ČēĶ¼ÖŠĆæĢõÕŪĻß±ķŹ¾ÖÜĘŚ±ķ¢ōA×唫¢÷A×åÖŠµÄijŅ»×åŌŖĖŲĒā»ÆĪļµÄ·Šµć±ä»Æ£¬ĆæøöŠ”ŗŚµć“ś±ķŅ»ÖÖĒā»ÆĪļ£¬ĘäÖŠaµć“ś±ķµÄŹĒ £Ø””””£©”£
A.H2S B.HCl
C.PH3 D.SiH4
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ŅŃÖŖA”¢B”¢C”¢DŗĶEĪåÖÖ·Ö×ÓĖłŗ¬Ō×ӵďżÄæŅĄ“ĪĪŖ1”¢2”¢3”¢4ŗĶ6£¬ĒŅ¶¼ŗ¬ÓŠ18øöµē×Ó£¬ÓÖÖŖB”¢CŗĶDÓÉĮ½ÖÖŌŖĖŲµÄŌ×Ó×é³É£¬ĒŅD·Ö×ÓÖŠĮ½ÖÖŌ×ÓøöŹż±ČĪŖ1”Ć1”£
(1)×é³ÉA·Ö×ÓµÄŌ×ÓµÄŗĖĶāµē×ÓÅŲ¼Ź½ŹĒ________£»
(2)BŗĶCµÄ·Ö×ÓŹ½·Ö±šŹĒ________ŗĶ________£»C·Ö×ÓµÄĮ¢Ģå½į¹¹³Ź
________ŠĪ£¬øĆ·Ö×ÓŹōÓŚ________·Ö×Ó(Ģī”°¼«ŠŌ”±»ņ”°·Ē¼«ŠŌ”±)£»
(3)ĻņDµÄĻ”ČÜŅŗÖŠ¼ÓČėÉŁĮæĀČ»ÆĢśČÜŅŗĻÖĻóŹĒ________________£¬øĆ·“Ó¦µÄ
»Æѧ·½³ĢŹ½ĪŖ_____________________________________________________”£
(4)Čō½«1 mol EŌŚŃõĘųÖŠĶźČ«Č¼ÉÕ£¬Ö»Éś³É1 mol CO2ŗĶ2 mol H2O£¬ŌņEµÄ
·Ö×ÓŹ½ŹĒ________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
¹ŲÓŚ¹¤ŅµÉś²śµÄŠšŹö£¬“ķĪóµÄŹĒ (””””)”£
A£®ÖĘĘÕĶز£Į§µÄÖ÷ŅŖŌĮĻŹĒ“æ¼ī”¢ŹÆ»ŅŹÆ”¢ŹÆÓ¢
B£®°±ŹĒÖĘ×÷µŖ·Ź”¢ĻõĖį”¢ļ§ŃĪµÄÖŲŅŖŌĮĻ
C£®½«¶žŃõ»ÆĮņ“ß»ÆŃõ»ÆÉś³ÉČżŃõ»ÆĮņŗó£¬ŌŚĪüŹÕĖžÄŚÓĆĖ®ĪüŹÕÖʵĆÅØĮņĖį
D£®ÖĘŌģĘÕĶØĖ®ÄąµÄÖ÷ŅŖŌĮĻŹĒš¤ĶĮ”¢ŹÆ»ŅŹÆ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
Ņ»¶ØĪĀ¶ČĻĀ£¬·“Ó¦X£Øg£©+3Y£Øg£©⇌2Z£Øg£©“ļµ½»ÆŃ§Ę½ŗāדĢ¬µÄ±źÖ¾ŹĒ£Ø””””£©
| ”” | A£® | X”¢Y”¢ZµÄ·Ö×ÓŹż±ČĪŖ1£ŗ3£ŗ2 |
| ”” | B£® | µ„Ī»Ź±¼äÉś³ÉamolX£¬Ķ¬Ź±Éś³É3a molY |
| ”” | C£® | X”¢Y”¢ZµÄÅضČĻąµČ |
| ”” | D£® | 3vÕż£ØX£©=vÄę£ØY£© |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com