


 �п�������㾫��ϵ�д�
�п�������㾫��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ����Ժǿ��������ˮ���������̱����ֽ�ˮ�����ۺ���̬���������빤�̽�����Э������ˮ��Դ��������Ϊ���ߣ��ѽ�ˮ�����ۡ���̬�����������ˮ��Ϊһ��������ϵͳ��������ԭ���ڵ�ˮ�����У���;��ҵ��ˮ�������ŷ������ˮ�ʶ�������������ij������Һ�У����д�����Mg2+��Al3+��Cu2+��Ag+���Է����ش��������⣺
����Ժǿ��������ˮ���������̱����ֽ�ˮ�����ۺ���̬���������빤�̽�����Э������ˮ��Դ��������Ϊ���ߣ��ѽ�ˮ�����ۡ���̬�����������ˮ��Ϊһ��������ϵͳ��������ԭ���ڵ�ˮ�����У���;��ҵ��ˮ�������ŷ������ˮ�ʶ�������������ij������Һ�У����д�����Mg2+��Al3+��Cu2+��Ag+���Է����ش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
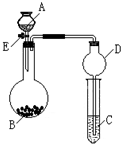
 ijͬѧ�������ͼ��ʾװ�ã��г�����ʡ�ԣ�����ϵ��ʵ�飬ʵ��ʱ��ҩƷA��μ��뵽����B�У����������ʵ��ش����⣺
ijͬѧ�������ͼ��ʾװ�ã��г�����ʡ�ԣ�����ϵ��ʵ�飬ʵ��ʱ��ҩƷA��μ��뵽����B�У����������ʵ��ش����⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
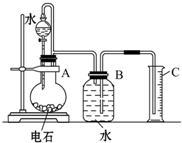 ijͬѧ�������ͼ��ʾ��ʵ��װ�������Եزⶨ��ʯ��̼���Ƶ�����������
ijͬѧ�������ͼ��ʾ��ʵ��װ�������Եزⶨ��ʯ��̼���Ƶ�����������| 0.29V |
| W |
| 0.29V |
| W |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
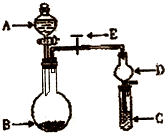 ��2010?����һģ����ѧʵ����һ��װ������������ɶ��ʵ�飬Aijͬѧ�������ͼ��ʾװ�ã��г�����ʡ�ԣ�����ϵ��ʵ�飮��ش�
��2010?����һģ����ѧʵ����һ��װ������������ɶ��ʵ�飬Aijͬѧ�������ͼ��ʾװ�ã��г�����ʡ�ԣ�����ϵ��ʵ�飮��ش��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| 1 | 10 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com