
| װ�� | ���� | ���ۼ����� |
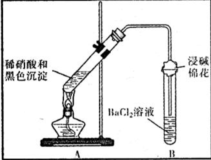 | ��A�Թ��к�ɫ�������ܽ� ��A�Թ��Ϸ����ֺ���ɫ���� ��B�Թ��г��ְ�ɫ���� | a�������˵����ɫ�������� ��ԭ���ԣ� b���Թ�B�в�����ɫ�������ܷ�Ӧ�����ӷ���ʽΪ NO2+SO2+Ba2++H2O�TBaSO4��+NO��+2H+ |
���� ��1������ͭ��Ũ���ᷴӦ���ɶ�������������н��
��2������������Ϣ�м���ͭ���ӵķ����Ԣڽ��з�����Ȼ��ó���ȷ���ۣ�
��3��a������ɫ����Ϊ����������˵��ϡ���ᱻ��ԭ����һ����������ɫ������л�ԭ�ԣ�
b�����ݷ�Ӧ����ۿ�֪��ɫ������ϡ���ᷴӦ�����˶�������֤����ɫ�����к�����Ԫ�أ�������������������Ļ�������ܹ����Ȼ�����Ӧ�������ᱵ�������ݴ�д����Ӧ�����ӷ���ʽ��
��4��CuS����Һ�д��ڳ����ܽ�ƽ�⣬����ƽ���ƶ�������
��5�����ݵζ�ʵ�����ݼ���ʣ�����������ʵ������õ�������ͭ����ͭ��Ӧ�ĸ���������ʵ��������ݷ�Ӧ�����ӷ���ʽ��ʽ����õ���
��� �⣺��1��Cu��Ũ���ᷴӦ��������ͭ�����������ˮ����ӦΪCu+2H2SO4��Ũ��$\frac{\underline{\;\;��\;\;}}{\;}$CuSO4+2SO2��+2H2O������������������SO2��
�ʴ�Ϊ��SO2��
��2������Һ�еμ�K4[Fe��CN��6]��Һ�����������ɫ������֤����Cu2+�����ݢڽ���ɫ��������ϡ�����У�һ��ʱ��μ�K4[Fe��CN��6]��Һ��δ�����ɫ������֪����ɫ������һ������CuO��
�ʴ�Ϊ����ɫ�����в�����CuO��
��3��a��A�Թ����Ϸ����ֺ���ɫ���壬˵����Ӧ����һ���������ɣ�֤���˺�ɫ������л�ԭ�ԣ��ڷ�Ӧ�б�������
�ʴ�Ϊ����ԭ�ԣ�
b�����ݷ�Ӧ�����B�Թ��г��ְ�ɫ������֪����ɫ����Ϊ���ᱵ��˵����ɫ�����к�����Ԫ�أ�������Ӧ�����ӷ���ʽΪ��NO2+SO2+Ba2++H2O�TBaSO4��+NO��+2H+��
�ʴ�Ϊ��NO2+SO2+Ba2++H2O�TBaSO4��+NO��+2H+��
��4��CuS������ˮ����ˮ��Һ�л��к�������Cu�ܽ⣬��Һ�д��ڳ����ܽ�ƽ�⣬CuS��s��?Cu2+��aq��+S2-��aq�����ȵ�Ũ���ὫS2-������ʹS2-Ũ�ȼ�С���ٽ�����ƽ���������ƶ���ʹCuS�ܽ⣻
�ʴ�Ϊ��CuS�����ܽ�ƽ��CuS��s��?Cu2+��aq��+S2-��aq�����ȵ�Ũ���ὫS2-������ʹS2-Ũ�ȼ�С���ٽ�����ƽ���������ƶ���ʹCuS�ܽ⣻
��5�������ķ�ӦΪ��
8MnO4-+5Cu2S+44H+�T10Cu2++5SO2��+8Mn2++22H2O
6MnO4-+5CuS+28H+�T5Cu2++5SO2��+6Mn2++14H2O
MnO4-+5Fe2++8H+�TMn2++5Fe3++4H2O
��Cu2S��CuS�����ʵ����ֱ�Ϊx��y��
��Cu2S��CuS��Ӧ��ʣ��KMnO4�����ʵ�����0.035L��0.1mol/L��$\frac{1}{5}$=0.0007mol��
160x+96y=0.2
$\frac{8x}{5}$+$\frac{6y}{5}$=0.04��0.075-0.0007
���x=0.0005mol��
Cu2S������������$\frac{0.0005mol��160g/mol}{0.2g}$��100%=40%��
�ʴ�Ϊ��40%��
���� ���⿼����Ũ����Ļ�ѧ���ʡ�����ʵ�鷽������ƣ���Ŀ�Ѷ��еȣ������漰�������Դ�֪ʶ��϶࣬����������Ϣ�ǽ���ؼ�������������ѧ���ķ���������������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | CaO+H2O�TCa��OH��2 | |
| B�� | Zn+2HCl�TZnCl2+H2�� | |
| C�� | NaCl+AgNO3�TAgCl+NaNO3 | |
| D�� | 2NaCl+2H2O$\frac{\underline{\;ͨ��\;}}{\;}$2NaOH+H2��+Cl2�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
| A�� | ������ˮ�������c��H+��•c��OH-��=10-20����Һ�У�Na+��Cl-��S2-��SO32- | |
| B�� | ��ɫ��Һ�У�K+��CH3COO-��HCO3-��MnO4- | |
| C�� | ����ʹ��̪��Һ������ɫ��Һ�У�Na+��CO32-��K+��ClO-��AlO2- | |
| D�� | ��������Һ��K+��Cl-��NO3-��Fe3+��SO42- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ֻ�Т٢ڢ� | B�� | ֻ�Т٢ڢۢݢ� | C�� | ֻ�Т٢ڢۢ� | D�� | �٢ڢۢܢݢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | pH=7����Һ�У�Fe3+��Cl-��Na+��NO3- | |
| B�� | ˮ�������[H+]=1��10-3mol/L ����Һ��Na+��CO32-��Cl-��K+ | |
| C�� | pH=1����Һ��NH4+��Cl-��Mg2+��SO42-�� | |
| D�� | Al3+��HCO3-��I-��Ca2+ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
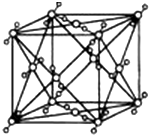 ��֪A��B��C��D��E�������ڱ��е�ǰ�����ڵ�Ԫ�أ����ǵĺ˵����A��B��C��D��E������B��D��Eԭ���������Ӳ��P�ܼ���������ϵĵ��Ӵ��ڰ����״̬��ͨ������£�A��һ�����������Ϊ�Ǽ��Է��ӣ��侧���ṹ����ͼ��ʾ��ԭ������Ϊ31��Ԫ���أ�Ga����Ԫ��B�γɵ�һ�ֻ������Ǽ���C����Ϊ�����ĵ�һ��뵼����Ϻ�GaEΪ�����ĵڶ����뵼�����֮���ڽ�10��Ѹ�ٷ�չ�����ĵ��������Ͱ뵼����ϣ�
��֪A��B��C��D��E�������ڱ��е�ǰ�����ڵ�Ԫ�أ����ǵĺ˵����A��B��C��D��E������B��D��Eԭ���������Ӳ��P�ܼ���������ϵĵ��Ӵ��ڰ����״̬��ͨ������£�A��һ�����������Ϊ�Ǽ��Է��ӣ��侧���ṹ����ͼ��ʾ��ԭ������Ϊ31��Ԫ���أ�Ga����Ԫ��B�γɵ�һ�ֻ������Ǽ���C����Ϊ�����ĵ�һ��뵼����Ϻ�GaEΪ�����ĵڶ����뵼�����֮���ڽ�10��Ѹ�ٷ�չ�����ĵ��������Ͱ뵼����ϣ��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �μ�KI-������Һ��Ϊ��ɫ | |
| B�� | ����Һ��Cu2+��NH4+��SO4-��Cl- ���Դ������� | |
| C�� | �������ữ��AgNO3��Һ��Ӧ�г������ɲ��ų����� | |
| D�� | ���Ƹ���Һʱ����FeBr2��ĩ�ܽ���HBr��Һ�У��������������� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com