��I������ʵ�������ȷ����
CDGH
CDGH
A������ˮ�Ҵ�������170��ʱ�������Ƶ���ϩ
B�����������еμ������ữ��AgNO
3��Һ���Լ������е���Ԫ��
C����ϡHNO
3��ϴ����������Ӧ���Թܣ�
D�����ӡ�Ũ������������ڷ�ˮԡ�м��ȿɵ÷�ȩ��֬
E���Ҵ����������2mol/L��������������Ƶ���������
F������Ũ��ˮ�����ۻ�ϣ���ȡ�屽
G���þƾ�ϴ��ʢ�Ź����ӵ��Թܣ�H������NaOH��Һϴ������֬���Թܣ�
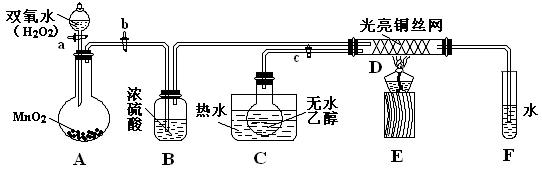
��II��ijʵ��С��������װ�ý����Ҵ���������ʵ�飮

��1��ʵ�������ͭ�����ֺ�ɫ�ͺ�ɫ�����������д���ɺ�ɫ�ֱ�Ϊ��ɫ����Ӧ��ѧ����ʽ����
CH
3CH
2OH+CuO
CH
3CHO+Cu+H
2O
CH
3CH
2OH+CuO
CH
3CHO+Cu+H
2O
���ڲ��Ϲ������������£�Ϩ��ƾ��ƣ���Ӧ���ܼ������У�˵�����Ҵ�����Ӧ��
����
����
��Ӧ��
��2����Ӧ����һ��ʱ����Թ�a�����ռ�����ͬ�����ʣ�������
��ȩ���Ҵ���ˮ
��ȩ���Ҵ���ˮ
������ƿ���ռ������������Ҫ�ɷ���
����
����
��
��3�����Թ�a���ռ�����Һ������ɫʯ����ֽ���飬��ֽ�Ժ�ɫ��˵��Һ���л�����
����
����
��Ҫ��ȥ�����ʣ������ڻ��Һ�м���
c
c
����д��ĸ����
a���Ȼ�����Һ����������b������������ c��̼��������Һ ��������d�����Ȼ�̼
Ȼ����ͨ��
����
����
��������������ƣ����ɳ�ȥ��