
��ҵ���ú�п����(��FeO��CuO������)���Ƶû���ZnO���������£�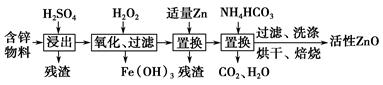
(1)���������У������õ���60%H2SO4(1.5 g��cm��3)����������H2SO4 100 mL��Ҫ18.4 mol��L��1��ŨH2SO4________ mL(����һλС��)��
(2)����������H2O2����Fe(OH)3�������֣�û��Cu(OH)2�������֣�����Һ��c(Fe3��)��2.6��10��18 mol��L��1������Һ��c(Cu2��)��ȡֵ��Χ��________mol��L��1��(��֪Ksp[Fe(OH)3]��2.6��10��39��
Ksp[Cu(OH)2]��2.2��10��20)
(3)����NH4HCO3�����ɵij�������̬��ΪZna(OH)b(CO3)c(a��b��cΪ������)�����ּ�ʽ̼��пA��B�Ļ���A��a��5��b��6�������ɼ�ʽ̼��пA�Ļ�ѧ����ʽΪ_______________________________________________��
(4)ȡϴ�ӡ���ɺ�ļ�ʽ̼��пA��B�Ļ����49.70 g�������ʵ���Ϊ0.10 mol�����±�����ȫ�ֽ�õ�37.26 g ZnO��3.584 L CO2(��״����)��ˮ��ͨ�����������ʽ̼��пB�Ļ�ѧʽ��
��(1)49.9(50.0Ҳ����)
(2)��2.2��10��6
(3)5ZnSO4��10NH4HCO3=Zn5(OH)6(CO3)2����5(NH4)2SO4��8CO2����2H2O
(4)������0.1 mol�������ȫ�ֽ�õ�ZnO��CO2��H2O�����ʵ����ֱ�Ϊ0.46 mol��0.16 mol��0.3 mol
��֪1 mol�������ƽ����4.6 mol Zn��1.6 mol C��6 mol H����֪1 mol A�к�HΪ6 mol����CΪ2 mol����1 mol B�к�HΪ6 mol����CΪ1 mol������B�Ļ�ѧʽ���Ա�ʾΪZnx(OH)6CO3���ɻ��ϼ۴�����Ϊ��ó�x��4����B�Ļ�ѧʽΪZn4(OH)6CO3(���������ⷨ����)
����



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�������洦����Ƥ�����ơ�ӡȾ�ȶ�������ɸ���Ⱦ�����۸������۸����Ըߣ����ױ����������������������
�Ź�ҵ�ϴ������Ժ�Cr2O72����ˮ�ķ������£�
����Cr2O72�������Է�ˮ�м���FeSO4��Һ��ʹCr2O72��ȫ��ת��ΪCr3����д���÷�Ӧ�����ӷ���ʽ�� ��
�ڵ�����Һ��pH��ʹCr3����ȫ������ʵ���Ҵ��Բⶨ��ҺpH�ķ���Ϊ ��25�棬��������Һ��pH=8������Һ�в���Cr3�������ʵ���Ũ��Ϊ mol/L������֪25��ʱ��Ksp[Cr(OH)3]=6.3��10��31��
�Ƹ�Ԫ����Ũ�ȵIJⶨ��ȷ��ȡ25.00mL��Cr2O72����Cr3�������Է�ˮ�������м���������(NH4)2S2O8��Һ��Cr3��������Cr2O72������г�ȥ������(NH4)2S2O8����������Һ�м��������KI��Һ����ַ�Ӧ���Ե���Ϊָʾ���������еμ�0.015mol/L��Na2S2O3����Һ���յ�ʱ����Na2S2O3��Һ20.00mL��
�����ˮ�и�Ԫ����Ũ�ȣ���λ��mg��L��1��д��������̣���
��֪�ⶨ�����з����ķ�Ӧ���£�
��2Cr3����3S2O82����7H2O =Cr2O72����6SO42����14H��
��Cr2O72����6I����14H��=2Cr3����3I2��7H2O
��I2��2S2O32��=2I����S4O62��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��һֻ���汻������10.2 g������������A������1 L 1.5 mol��L��������������Һ�У���һ��ʱ���ȡ����������Һ��������12.6�ˣ�����Һ��Ϊ����Һ�������Ϊ1 L���ٰ���һֻ����Ҳ������һ������������������B������1 L 0.9 mol��L��������Һ�У�����һ��ʱ��ȡ��������������25.8�ˣ���Һ��������25.2�ˣ�����Һ��Ϊ����Һ�������Ϊ1 L���ش��������⣺
��1������Һ������ ��д��ѧʽ���������ʵ����� ;
��2������Һ������ ��д��ѧʽ���������ʵ����� ;
��3������������Һ��Ӧ����ʹ�μӵ�����С������������࣬Ӧ�� ��Һ ����ס��ң�L �μӵ� ��Һ�У���ס��ң���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
WO3�������Ʊ�����Ԫ���������������ȡ��乤ҵ�����������£�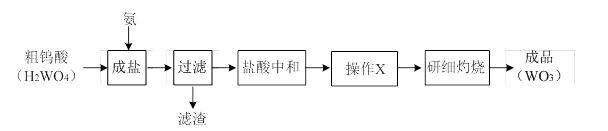
��1������X��Ŀ����Ϊ�˻�ô�����������茶��壬�ò������������������кͺ����Һ ����ȴ�ᾧ�� �����º�ɡ�
��2��ʵ�ʹ�ҵ�����У���������茶��壨������NH4Cl���壩�ɲ����ᴿ��ֱ�����գ���ԭ���� ��
��3����֪��������茶���[x(NH4)2O��yWO3��zH2O]���ȷֽ�Ļ�ѧ����ʽ���£�
x(NH4)2O��yWO3��zH2O��WO3 +NH3��+H2O��(δ��ƽ)��
ijͬѧΪ�ⶨ������茶������ɣ���������ʵ�飺
��ȷ��ȡ16.21g��Ʒ����ϸ���գ�
�ڽ�����������ͨ��װ�м�ʯ�Ҹ���ܣ�������ճƵø��������1.44g��
�۳�����ȴ��Ĺ�������Ϊ13.92g��
ͨ������ȷ����������茶���Ļ�ѧʽ��д��������̣���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������һ����Ҫ�Ĺ�ҵ��Ʒ��Ҳ��һ����Ҫ�Ļ���ԭ�ϡ�Ϊ�о��������Ʊ������ʣ�ijѧϰС������������̽�����
��1��ʵ����������������ʯ�����Ȼ���Ʊ�������д���÷�Ӧ�Ļ�ѧ����ʽ��ע����Ӧ������__________________________________________________��
��2��ʵ�����л����Բ�����ͼװ�ã���ȥ�˼г�װ�ã���ҩƷ�����Ʊ�������
�ټ�ͬѧ�����÷������Ʊ�������ԭ��������ѧϰС���ڳ�Աչ�������ۣ��ó�������Ľ��ۣ����в���ȷ����________������ĸ��ţ���
a����ˮ�е�NH3��H2O�ֽ�
b����ˮ�д��ڿ��淴Ӧ��NH3��H2O  NH4+��OH����NaOH����ʹ�ÿ��淴Ӧ��ƽ�������ƶ�
NH4+��OH����NaOH����ʹ�ÿ��淴Ӧ��ƽ�������ƶ�
c����Ӧԭ������Ρ���ʯ���Ʊ������ķ�Ӧԭ����ͬ
d��NaOH����ˮ�ų�������ʹNH3��H2O�ֽ�
����ͬѧȡ��������Ϊ34%��0.89 g��mL��1��Ũ��ˮ10 mL���ù�����NaOH��֮��ϣ�Ȼ����500 mL����ƿ�ռ�������������������ռ�����״���µİ���________����ƿ��
��3����ͬѧ���а�������Ȫʵ��̽����
���ռ�����ʱ��Ҫ�ø���������ͼ��װ��B��ʢװ�ĸ������________��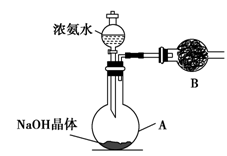
�ڸ�ͬѧ����ͼװ�ã��ɹ����������Ȫʵ�顣�����Ҫ��д����ͬѧ��������ȷ������____________________________________________________��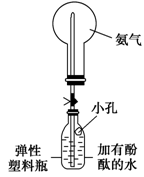
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���ʵ����Ǹ��л�ѧ�г��õ�������������������й������ݵļ��㡣
��1��0.2 g H2���и�______Hԭ�ӡ�
��2����״���£�������ͬ��ԭ������CO��CO2�����֮��Ϊ______��
��3��100 mL ijAl2��SO4��3��Һ�У�n��Al3+��="0.20" mol��������c��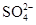 ��= ______mol/L��
��= ______mol/L��
��4����9.5 gij���۽������Ȼ����к�0.2 mol Cl-�����Ȼ����Ħ������Ϊ______���ý���Ԫ�ص����ԭ������Ϊ______��
��5����״����6.72 L CO��һ������ Fe2O3ǡ����ȫ��Ӧ������Fe��CO2����ʣ����������Ϊ______g��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ʵ����Ҫ����1mol/L��ϡ����250mL���ش��������⣺
��1����Ҫ98%�ܶ�Ϊ1.84g/cm3��Ũ���� mL
��2������ʱ������ʹ�õ������� ���������� (�����) ��ȱ�ٵ������� ������ ��
���ձ�����100 mL��Ͳ������20 mL��Ͳ ��1000 mL����ƿ ��250 mL����ƿ����������ƽ(������) �߲�����
��3������ʱ����ʵ�������õ��������������÷ֱ��� �� ��
��4�����ƹ����г��������������������ҺŨ���к�Ӱ�죨�ƫ�ߡ���ƫ�͡�����Ӱ�족��
��û��ϴ���ձ��Ͳ���������������������������
�������ˮ�����˿̶��ߣ�ȡ��ˮʹҺ��ǡ�õ��̶��ߡ�����������������
������ƿû�и������������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��2.3����Ͷ�뵽ˮ�����з�̪���У���Ӧ��������Һ��0.1������
��1����Ӧ�����У����Է����Ƹ���ˮ�棬˵���Ƶ��ܶ� ������ڡ���С�ڡ�����
�ڡ���ˮ���ܶȣ���Һ����� ɫ��
��2����ѧ��Ӧ����ʽ�� ��
��3���������Һ�����ʵ���Ũ���Ƕ��٣���д��������̣�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ʵ������Ҫ0.80 mol��L��1 NaOH��Һ475 mL ��0. 40 mol��L��1����500 mL��������������Һ����������ش��������⣺
(1)��ͼ��ʾ��������������Һ�϶�����Ҫ����__________(����)������������Һ�����õ��IJ���������__________(����������)��
(2)����ƿ�����߱��Ĺ�����__________(����)��
| A������һ�����ȷŨ�ȵı���Һ |
| B����ȡһ�������Һ�� |
| C����������ƿ������µ����������Һ�� |
| D��������Һ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com