
øł¾ŻČČ»Æѧ·½³ĢŹ½£ŗS(l)£«O2(g)===SO2(g)””¦¤H£½£293.23 kJ”¤mol£1 £¬·ÖĪöĻĀĮŠĖµ·ØÖŠÕżČ·µÄŹĒ(””””)
A£®S(s)£«O2(g)===SO2(g)£¬·“Ó¦·Å³öµÄČČĮæ“óÓŚ293.23 kJ”¤mol£1
B£®S(g)£«O2(g)===SO2(g)£¬·“Ó¦·Å³öµÄČČĮæŠ”ÓŚ293.23 kJ”¤mol£1
C£®1 mol SO2(g)µÄ¼üÄܵÄ×ÜŗĶ“óÓŚ1 mol S(l)ŗĶ1 mol O2(g)µÄ¼üÄÜÖ®ŗĶ
D£®1 mol SO2(g)µÄ¼üÄܵÄ×ÜŗĶŠ”ÓŚ1 mol S(l)ŗĶ1 mol O2(g)µÄ¼üÄÜÖ®ŗĶ
 ÅąÓÅŗĆ¾ķµ„ŌŖ¼ÓĘŚÄ©¾ķĻµĮŠ“š°ø
ÅąÓÅŗĆ¾ķµ„ŌŖ¼ÓĘŚÄ©¾ķĻµĮŠ“š°ø Ņ»ĻßĆūŹ¦ČØĶž×÷Ņµ±¾ĻµĮŠ“š°ø
Ņ»ĻßĆūŹ¦ČØĶž×÷Ņµ±¾ĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
A”¢B”¢C”¢D”¢E¾łĪŖÓŠ»śĪļ£¬ĖüĆĒ¾ßÓŠĻĀĶ¼µÄ×Ŗ»Æ¹ŲĻµ£ŗ
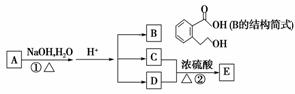
ŅŃÖŖ£ŗCÄÜÓėNaHCO3·¢Éś·“Ó¦£¬C”¢DĻą¶Ō·Ö×ÓÖŹĮæĻąµČ£¬EÄÜ·¢ÉśŅų¾µ·“Ó¦£¬Ļą¶Ō·Ö×ÓÖŹĮæĪŖ74”£
Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
(1)Š“³öCµÄĆū³Ę£ŗ________£¬EÖŠŗ¬ÓŠµÄ¹ŁÄÜĶÅ(Š“½į¹¹¼ņŹ½)________”¢________£»Š“³öÓŠ»śĪļBæÉÄÜ·¢ÉśµÄĮ½ÖÖ·“Ó¦ĄąŠĶ£ŗ_______________________£»
(2)Š“³ö·“Ó¦¢ŚµÄ»Æѧ·½³ĢŹ½£ŗ_____________________________________£»
øĆ·“Ó¦Šč¼ÓČȵÄÄæµÄŹĒ___________________________________________£»
(3)AµÄ½į¹¹¼ņŹ½ĪŖ________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ÓŠ»ś»ÆŗĻĪļÓŠ²»Ķ¬µÄ·ÖĄą·½·Ø£¬ĻĀĮŠĖµ·ØÕżČ·µÄŹĒ(””””)
¢Ł“Ó×é³ÉŌŖĖŲ·Ö£ŗĢž£¬ĢžµÄŃÜÉśĪļ””¢Ś“Ó·Ö×ÓÖŠĢ¼¹Ē¼ÜŠĪד·Ö£ŗĮ“דӊ»ś»ÆŗĻĪļ£¬»·×“ÓŠ»ś»ÆŗĻĪļ””¢Ū“Ó¹ŁÄÜĶÅ·Ö£ŗĻ©Ģž”¢Č²Ģž”¢·¼ĻćĢž”¢Ā±“śĢž”¢“¼”¢·Ó”¢Č©”¢ĶŖ”¢ōČĖį”¢õ„µČ
A£®¢Ł¢Ū B£®¢Ł¢Ś
C£®¢Ł¢Ś¢Ū D£®¢Ś¢Ū
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
A”«GŹĒ¼øÖÖĢžµÄ·Ö×ÓĒņ¹÷Ä£ŠĶ(ČēĶ¼)£¬¾Ż“Ė»Ų“šĻĀĮŠĪŹĢā£ŗ
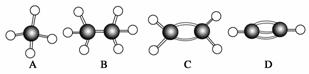
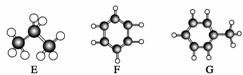
(1)³£ĪĀĻĀŗ¬Ģ¼Įæ×īøßµÄĘųĢ¬ĢžŹĒ
________________________________________________________________________
(Ģī×ÖÄø)”£
(2)Äܹ»·¢Éś¼Ó³É·“Ó¦µÄĢžÓŠ________ÖÖ”£
(3)Ņ»Ā±“śĪļÖÖĄą×ī¶ąµÄŹĒ________(ĢīŠ“×ÖÄø)”£
(4)Š“³öŹµŃéŹŅÖĘČ”DµÄ»Æѧ·½³ĢŹ½
________________________________________________________________________
________________________________________________________________________ӣ
(5)Š“³öF·¢ÉśĻõ»Æ·“Ó¦µÄ»Æѧ·½³ĢŹ½
________________________________________________________________________
________________________________________________________________________ӣ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ŅŃÖŖµØ·ÆČÜÓŚĖ®Ź±ČÜŅŗĪĀ¶Č½µµĶ”£µØ·Æ·Ö½āµÄČČ»Æѧ·½³ĢŹ½ĪŖCuSO4”¤5H2O(s)===CuSO4(s)£«5H2O(l)””¦¤H£½£«Q1 kJ”¤mol£1”£ŹŅĪĀĻĀ£¬Čō1 molĪŽĖ®ĮņĖįĶČܽāĪŖČÜŅŗ·ÅČČQ2 kJ£¬Ōņ(””””)””””””””””””””””””””””””””””””””””
A£®Q1>Q2 B£®Q1£½Q2 C£®Q1<Q2 D£®ĪŽ·Ø±Č½Ļ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ŅŃÖŖ25”ꏱ£¬ŗĻ³É°±·“Ó¦N2(g)£«3H2(g) 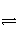
 2NH3(g)
2NH3(g)
¦¤H£½£92.0 kJ”¤mol£1£¬½«“ĖĪĀ¶ČĻĀµÄ1 mol N2ŗĶ3 mol H2·ÅŌŚŅ»ĆܱÕČŻĘ÷ÖŠ£¬ŌŚ“߻ƼĮ“ęŌŚŹ±½ųŠŠ·“Ó¦”£²āµĆ·“Ó¦·Å³öµÄČČĮæĪŖ23 kJ(¼Ł¶Ø²āĮæ¹ż³Ģ֊ƻӊÄÜĮæĖšŹ§)£¬ŌņN2µÄ×Ŗ»ÆĀŹĪŖ(””””)
A£®25% B£®50% C£®75% D£®ĪŽ·ØČ·¶Ø
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ŗģĮ×P(s)ŗĶCl2(g)·¢Éś·“Ӧɜ³ÉPCl3(g)ŗĶPCl5(g)”£·“Ó¦¹ż³ĢŗĶÄÜĮæ¹ŲĻµČēĻĀĶ¼ĖłŹ¾(Ķ¼ÖŠµÄ¦¤H±ķŹ¾Éś³É1 mol²śĪļµÄŹż¾Ż)”£
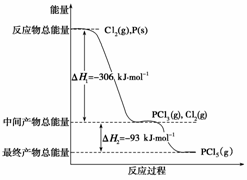
øł¾ŻÉĻĶ¼»Ų“šĻĀĮŠĪŹĢā£ŗ
(1) PŗĶCl2·“Ӧɜ³ÉPCl3µÄČČ»Æѧ·½³ĢŹ½ŹĒ
________________________________________________________________________
________________________________________________________________________ӣ
(2) PCl5·Ö½ā³ÉPCl3ŗĶCl2µÄČČ»Æѧ·½³ĢŹ½ŹĒ
________________________________________________________________________
________________________________________________________________________ӣ
ÉĻŹö·Ö½ā·“Ó¦ŹĒŅ»øöæÉÄę·“Ó¦”£ĪĀ¶ČT1Ź±£¬ŌŚĆܱÕČŻĘ÷ÖŠ¼ÓČė0.80 mol PCl5£¬·“Ó¦“ļµ½Ę½ŗāŹ±PCl5»¹Ź£0.60 mol£¬Ęä¦Į1µČÓŚ________£»Čō·“Ó¦ĪĀ¶ČÓÉT1Éżøßµ½T2£¬Ę½ŗāŹ±PCl5µÄ·Ö½āĀŹĪŖ¦Į2£¬¦Į2________¦Į1(Ģī”°“óÓŚ”±”¢”°Š”ÓŚ”±»ņ”°µČÓŚ”±)”£
(3) ¹¤ŅµÉĻÖʱøPCl5Ķس£·ÖĮ½²½½ųŠŠ£¬ĻČ½«PŗĶCl2·“Ӧɜ³ÉÖŠ¼ä²śĪļPCl3£¬Č»ŗó½µĪĀ£¬ŌŁŗĶCl2·“Ӧɜ³ÉPCl5”£ŌŅņŹĒ__________________________________________
______________________ӣ
(4)PŗĶCl2·ÖĮ½²½·“Ӧɜ³É1 mol PCl5µÄ¦¤H3£½______£¬PŗĶCl2Ņ»²½·“Ӧɜ³É1 mol PCl5µÄ¦¤H4________¦¤H3(Ģī”°“óÓŚ”±”¢”°Š”ÓŚ”±»ņ”°µČÓŚ”±)”£
(5)PCl5Óė×ćĮæĖ®³ä·Ö·“Ó¦£¬×īÖÕÉś³ÉĮ½ÖÖĖį£¬Ęä»Æѧ·½³ĢŹ½ŹĒ
________________________________________________________________________ӣ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
¶ĢÖÜĘŚŌŖĖŲ¼×”¢ŅŅ”¢±ū”¢¶”µÄŌ×ÓŠņŹżŅĄ“ĪŌö“󣬼×ŗĶ¶”µÄŌ×ÓŗĖĶā¾łÓŠĮ½øöĪ“³É¶Ōµē×Ó£¬ŅŅ”¢±ū”¢¶”×īøß¼ŪŃõ»ÆĪļ¶ŌÓ¦µÄĖ®»ÆĪļĮ½Į½Ö®¼äÄÜĻą»„·“Ó¦”£ĻĀĮŠĖµ·Ø“ķĪóµÄŹĒ£Ø £©
A£®ŌŖĖŲ±ūµÄµ„ÖŹæÉÓĆÓŚŅ±Į¶½šŹō B£®¼×Óė¶”ŠĪ³ÉµÄ·Ö×ÓÖŠÓÉ·Ē¼«ŠŌ·Ö×Ó
C£®¼ņµ„Ąė×Ó°ė¾¶£ŗ¶” > ŅŅ > ±ū D£®¼×ÓėŅŅŠĪ³ÉµÄ»ÆŗĻĪļ¾łÓŠŃõ»ÆŠŌ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĶ¼ĖłŹ¾×°ÖĆÖŠ£¬¼×”¢ŅŅ”¢±ūČżøöÉÕ±ŅĄ“Ī·Ö±šŹ¢·Å100 g 5.00%µÄNaOHČÜ
Ņŗ”¢×ćĮæµÄCuSO4ČÜŅŗŗĶ100 g 10.00%µÄK2SO4ČÜŅŗ£¬µē¼«¾łĪŖŹÆÄ«µē¼«”£
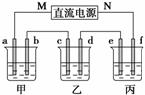
(1)½ÓĶصēŌ“£¬¾¹żŅ»¶ĪŹ±¼äŗ󣬲āµĆ±ūÖŠK2SO4ÖŹĮæ·ÖŹżĪŖ10.47%£¬ŅŅÖŠcµē¼«ÖŹĮæŌö¼Ó”£¾Ż“Ė»Ų“šĪŹĢā£ŗ
¢ŁµēŌ“µÄN¶ĖĪŖ________¼«£»
¢Ł µē¼«bÉĻ·¢ÉśµÄµē¼«·“Ó¦ĪŖ___________________________________________£»
¢ŪĮŠŹ½¼ĘĖćµē¼«bÉĻÉś³ÉµÄĘųĢåŌŚ±ź×¼×“æöĻĀµÄĢå»ż£ŗ________L£»
¢Üµē¼«cµÄÖŹĮæ±ä»ÆŹĒ__________g£»
¢Żµē½āĒ°ŗóø÷ČÜŅŗµÄpHŹĒ·ń·¢Éś±ä»Æ£ŗ
¼×ČÜŅŗ________________£»ŅŅČÜŅŗ________________£»
±ūČÜŅŗ________________£»
(2)Čē¹ūµē½ā¹ż³ĢÖŠĶČ«²æĪö³ö£¬“ĖŹ±µē½āÄÜ·ń¼ĢŠų½ųŠŠ£¬ĪŖŹ²Ć“£æ
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com