
| ������ | Al(OH)3 | Fe(OH)3 | Fe(OH)2 | Cu(OH)2 | Zn(OH)2 |
| pH | 5.2 | 3.2 | 9.7 | 6.7 | 8.0 |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 2RAn���л��㣩 + nH2SO4 (ˮ��)Ϊ��ߢ�����ȡ�ٷ��ʣ�Ӧ��ȡ�Ĵ�ʩ�� ��
2RAn���л��㣩 + nH2SO4 (ˮ��)Ϊ��ߢ�����ȡ�ٷ��ʣ�Ӧ��ȡ�Ĵ�ʩ�� ��| pH | 1.3 | 1.4 | 1.5 | 1.6 | 1.7 | 1.8 | 1.9 | 2.0 | 2.1 |
| ��������% | 88.1 | 94.8 | 96.5 | 98.0 | 98.8 | 98.8 | 96.4 | 93.1 | 89.3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
| A��CaO | B��CaCO3 | C��NH3��H2O | D��Ba(OH)2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
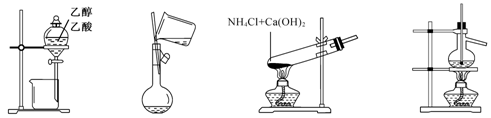
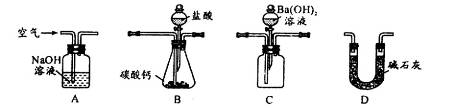
| A����ͼ1��ʾװ�÷����Ҵ������� |
| B����ͼ2��ʾ��װ��������ƿ��ת��Һ�� |
| C����ͼ3��ʾ��װ���Ʊ��������� |
| D����ͼ4��ʾ��װ�÷���ʯ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
| | �� | �� | �屽 |
| �ܶ�/g��cm-3 | 0. 88 | 3. 10 | 1. 50 |
| �е㣯�� | 80 | 59 | 156 |
| ˮ���ܽ�� | �� | �� | �� |
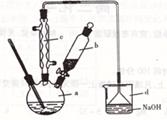
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
| ʵ�鲽�� | Ԥ������ͽ��� |
| ����1����������ĥ���ݡ����˵õ����������Һ�� | |
| ����2��������Һ�������ԣ��μ�����CaCl2��Һ�� | ���ְ�ɫ������˵�������п��ܺ��в����κ�̼���Ρ� |
| ����3��ȡ����2�ij������Թ��У� | |
| ����4�� | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A�������յķ�����������˿�Ͳ�˿ |
| B����ˮ���𱽺��Ҵ� |
| C��ʵ������N2��H2�Ʊ�NH3 |
| D���ñ���NaHCO3��Һ��ȥCO2�л��е�SO2���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
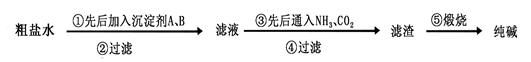
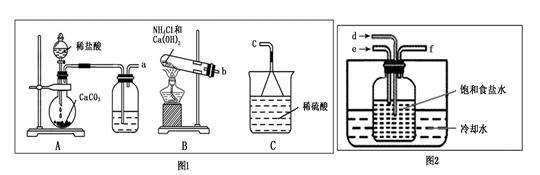
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��ϡ���� | B��ˮ | C���Ȼ�����Һ | D��̼������Һ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com