
����ʯ�ǹ�ҵ��������Ҫԭ��֮һ������Ҫ�ɷ�Ϊ����������(�������в�����Ԫ�غ���Ԫ�أ������ʲ������ᷴӦ)��ij�о���ѧϰС���ij����ʯ������������Ļ�ѧʽ����̽����
��.����ʯ�к������IJⶨ������ʵ����̲��������벹��������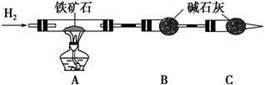
(1)����ͼ��װ��������______________________________________________��
(2)��8.0 g����ʯ����Ӳ�ʲ������У�װ��B��C�е�ҩƷ��ͼ��ʾ(�г�������ʡ��)��
(3)����˵����ܿڴ����ϵػ���ͨ��H2��____________________________��
��ȼA���ƾ��ƣ�
(4)��ַ�Ӧ�����ƾ��ƣ�________________________________________��
(5)��÷�Ӧ��װ��B����2.25 g��������ʯ�����İٷֺ���Ϊ________��
��.����ʯ�к������IJⶨ���������¡�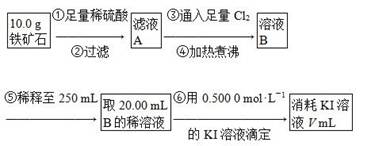
(1)���������������___________________________________________��
(2)��������õ��IJ����������ձ�����ͷ�ιܡ�250 mL����ƿ��________��
(3)�����йز���IJ�����˵����ȷ����________��
a����Ϊ��ˮΪ��ɫ�����Եζ������в����ָʾ��
b����ƿ����Ҫ�ô���Һ��ϴ
c���ζ������п����õ�����Һ��ָʾ��
d���ζ������У��۾�ע�ӵζ�����Һ��仯
e���ζ�������30 s����Һ���ָ�ԭ������ɫ���ٶ���
f���ζ������ζ��ܼ��첿�������ݣ���ⶨ���ƫ��
(4)���ζ�����������0.500 0 mol��L��1 KI��Һ20.00 mL��������ʯ�����İٷֺ���Ϊ________��
��.�ɢ���������������ʯ������������Ļ�ѧʽΪ________��
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
�������ƣ�NaN3����������ȫ�����е���Ҫ�ɷ֣����ڷ�����ײ��˲��ֽ�����������彫���ҹ���ʵ���Ҳⶨ����������Ʒ��NaN3������������ʵ�鲽�����£�
�ٳ�ȡԼ2.5000g�����������������250mL��Һ��
��ȷ��ȡ25.00mL��Һ������ƿ�У��õζ��ܼ���50.00mL 0.1000mol��L��1
(NH4)2Ce(NO3)6����������泥���
[������ӦΪ��2(NH4)2Ce(NO3)6 +2NaN3=4NH4NO3+2Ce(NO3)3+2NaNO3+3N2��]�����ʲ����뷴Ӧ����
�۷�Ӧ����Һ��ϡ�ͣ�Ȼ������Һ�м���5mLŨ���ᣬ����2���ڷ�����ָʾҺ����0.0500mol��L��1(NH4)2Fe(SO4)2����������泥����ζ���Һ�ζ�������Ce4+����Һ�ɵ���ɫ��Ϊ�ƺ�ɫ�������ķ�ӦΪ��Ce4++Fe2+= Ce3++Fe3+����������������隣���Һ24.00mL��
��1����������Ƶ���������Һʱ�������õ��ձ�������������Ͳ�⣬���õ��IJ��������� �� ��
��2������������ײ��ʱ�������ֵ��ʣ���Ӧ�Ļ�ѧ����ʽΪ ��
��3��������������ȷ���ζ����յ��ȡ�ζ����� (NH4)2Fe(SO4)2����Һ�����ͼͼʾ��ȡ�����������ⶨ��Ʒ�е��������������� ��ѡ���ƫ����ƫС�����䡱����
��4��ͨ������ȷ���������������к�NaN3����������Ϊ���٣�д��������̣���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ʵ������Ũ������ͭ�ķ�Ӧ��ȡ����NaHSO3��ʵ��װ����ͼ��ʾ��
��1��װ���ҵ�������_____________________��
��2��װ�ö���������������Ⱦ������SO2���壬�䷴Ӧ�����ӷ���Ϊ ��
��3�� SO2 ������Ư���ԡ���ԭ�Ժ������ԡ���SO2 ͨ����ˮ�У�SO2���ֵ���________�ԣ���ѧ��Ӧ����ʽΪ ��
��4����Ӧ�����У������еι����Ʒ����Һ������ƿ��������Ϊ ������Һ�е�NaOH��ȫת��Ϊ��NaHSO3��
��5��������û�м���Ʒ����Һ������ȷ�ж����������Ƿ���ȫת�������пɹ�ѡ����������Լ����ձ����Թܡ�����������ͷ�ιܣ� 2 mol/L���ᡢ2 mol/L���ᡢ1 mol/L�Ȼ�����Һ��l mol/L����������Һ��Ʒ����Һ������ˮ��
�����ʵ��̽�����պ�������Ƿ����NaHSO3 ��Na2SO3����ʵ�������Ԥ�ڵ�ʵ������ͽ��������±��С�
| ʵ����� | Ԥ����������� |
| ����1��ȡ��������Һ�����Թ��У��μӹ���lmol/L�Ȼ�����Һ������һ��ʱ��õ���ҺA����B�� | |
| ����2��������B�м�������ˮϴ�ӳ��������ú���ȥ�ϲ���Һ����������2�Σ���������Ʒ�죬�� | ��Ʒ����ɫ���������ݣ����� |
| ����3�� | �� �� �� ���� �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��1��������ͭ������ᾧˮ�����IJⶨʵ���У�������������Ҫ����
�Ρ�
��2���к��Ȳⶨ��ʵ���У��õ��IJ����������ձ����¶ȼơ� �� ��
��3����������ѧ��ѧ�г��ò���������ɵ�ʵ��װ��ͼ(������Ҫ�������м���Һ������)��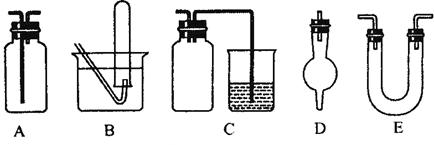
��ش��������⣺
�����������ﰱ����װ����_______________(����ĸ)��
�ڼ��������ռ��������������ռ�һ�����������װ����_______________(����ĸ)��
����ʵ�����Ʊ�������ʵ���У����Գ�ȥ�������Ȼ�������������װ����________________ (����ĸ)��
����������ϩ����ˮ��Ӧ�ƶ��������ʵ��װ����__________(����ĸ)��
����Cװ���У������ձ��ڵ�����������Һ����β��������������ƿ��������___________________��
��4��Ϊ�Ƚ�Fe3+��Cu2+��H2O2�ֽ�Ĵ�Ч����ijͬѧ�������ͼ��ʾ��ʵ�顣
�ٿ�ͨ���۲� �����Եĵó����ۣ�
����Aͬѧ�����CuSO4��ΪCuCl2��Ϊ�������������� ��
��������Aͬѧ�ĸĽ�����������Ϊ��������θĽ��� ��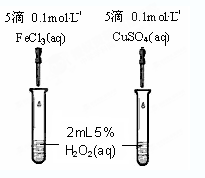
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ʴ���
( 14��)ij��ѧС�����Na2O2��ˮ��Ӧ��ʵ�飬����ͼ��ʾ����С����Թ�c�к�ɫ��ȥ��ԭ�����̽����
��1��Na2O2�к��еĻ�ѧ�����ͣ� ����д��a�з�Ӧ�Ļ�ѧ����ʽ ��
�������ϣ�
�ٵ�NaOH��ҺpH��13ʱ������ʹ��̪�ɺ�ɫ��Ϊ��ɫ��
��Na2O2��ˮ��Ӧ���������У�Na2O2 + H2O =" NaOH" + H2O2 2H2O2 = 2H2O + O2��
��2�������ʵ����֤Na2O2��ˮ��Ӧ�����Һ����H2O2������ȡ����b��Һ���Թ��У� ��֤����Һ����H2O2������
��3��������ϣ���С��ͬѧ��c����Һ��ɫ��ȥ��ԭ��������¼��裺
�� ��
�� ��Һ��H2O2�ƻ���̪�Ľṹ��
�� NaOH��H2O2��ͬ���ý����
��4����С��ͬѧ���c����Һ��pHΪ14����Ϊ�������ų�����ڡ��ۣ������ֽ���������ʵ�飬������±��հ״���
| ʵ�� | ���� | ���� | ���� |
| 1 | ������H2O2�еμ�2�η�̪������һ��ʱ�䣬�ټ���NaOH��Һ��pH=12 | ����NaOH����ɫ��Һ�ȱ�죬����ɫ | �� |
| 2 | ������ NaOH��Һ��pH=14���еμ�2�η�̪���ټ�����ϡ��������Һ pH=12 | ��Һ�ȱ�죬����ɫ����������ֳ��ֺ�ɫ���Ҳ���ɫ |  |
| 3 | ��Na2O2��ˮ��Ӧ�����Һ��pH=14���еμ�2�η�̪���ټ�����ϡ��������Һ pH=12 ���� | �� ���� | ��ҺpH����13ʱ��NaOHʹ������Һ��ɫ��pH��8~13ʱ�� NaOH��H2O2��ͬ����ʹ��Һ��ɫ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
(15��)������ʾ��þ�뱥��̼��������Һ��Ӧ������������Ͱ�ɫ�����ijͬѧͨ������ʵ��̽����Ӧԭ������֤���
ʵ��I����ɰֽ��ȥþ����������Ĥ���������ʢ�������з�̪�ı���̼��������Һ���ձ��У�Ѹ�ٷ�Ӧ�������������ݺͰ�ɫ�������Һ��dz��ɫ���
��1����ͬѧ�Է�Ӧ�в����İ�ɫ�������������²²⣺
�²�1����ɫ���������Ϊ����������
�²�2����ɫ���������ΪMgCO3
�²�3����ɫ���������Ϊ��ʽ̼��þ[xMg(OH)2?yMgCO3]
��2��Ϊ��ȷ������ɷ�(�������������塢��ɫ�����P��Һ������)���������¶���ʵ�顣����д���пհף�
| ʵ����� | ʵ �� | ʵ������ | �� �� |
| ʵ��� | ��ʵ��I���ռ����������ȼ | ����ȼ�գ�����ʵ���ɫ | ����ɷ�Ϊ�� �� |
| ʵ��� | ��ʵ��I�еİ�ɫ�������˳���ϴ�ӣ�ȡ������������ �� | �� | ��ɫ�������к���MgCO3 |
| ʵ��� | ȡʵ����е���Һ�������м����� ���� ���� ϡ��Һ | ������ɫ��������Һ��ɫ��dz | ��Һ�д���CO32������ |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
Ϊ��̽��SO2��Na2O2�ķ�Ӧ�Ƿ�������CO2����ͬѧ�������ͼʵ��װ�á��ش��������⣺
��1���ƿ������������ǵ�ľ������C�Թܿڣ�δ��ľ����ȼ����ͬѧ�����ΪSO2��Na2O2�ķ�Ӧ��ͬ��CO2���밴��ͬѧ�Ĺ۵�д����Ӧ�Ļ�ѧ����ʽ ��
��2����ͬѧ��Ϊ���۷�Ӧԭ����Σ����ն���O2��������ͬѧ�������� ��
������ͬѧ�Ĺ۵㣬��װ�������ĸĽ��ǣ� ��
��3������Na2O2 ��ȫ��Ӧ����Ӧ��Bװ���й�������������ǣ�
�� Na2SO3�� �� Na2SO4�� �� Na2SO3��Na2SO4
�����ʵ�鷽�����飬д��ʵ�鲽���Լ�Ԥ������ͽ��ۣ�����±���
��ѡ�Լ���2 mol��L��1 HCl�� 1mol��L��1HNO3�� 1 mol��L��1 BaCl2�� 1 mol��L��1 Ba(NO3)2��
0.01mol��L��1KMnO4������Һ��
| ʵ�鲽�� | Ԥ������ͽ��� |
| ����1��ȡB�е�����������Ʒ���Թ��У��μ���������ˮ���ܽ⣬Ȼ��ȡ��������Һ�ֱ����ڢ��Թ��С� | ������ȫ�ܽ� |
| ����2�������Թ��м��� ���ٵμ� �� | ����֤������ ���к�Na2SO4�� |
| ����3�������Թ��� �� | �� , ��˵������������Na2SO3���� , ��˵����������û��Na2SO3�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
ҽ���ϸ�������Һ�������������õ�����
| A�������� | B����ѿ�� | C������ | D����ά�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
��ɽ�緢ʱ�������Ļ�ɽ������Ҫ����ˮ������CO2��CO��SO2��SO3��H2S��HCl��Cl2��HF�����ʡ���Щ������Զ�������ɼ������ص�Σ�������ж���Щ������˺Ͷ�����ɵ�Σ����������ȷ���ǣ� ��
| A����CO2����ɵ�����ۼ��ڻ�ɽ�ڸ����ĵ��ݵ����������γɡ���������ʹ������ �еĶ�����Ϣ���� |
| B��SO2��SO3��H2S�Ⱥ�����������γ������⣬����ֲ���뽨������Ӱ�죬�䱾 ���Ķ��Բ��� |
| C������CO�Ĵ��ڣ���ʹ�˻���ѪҺ�е�Ѫ�쵰�������������ͣ��Ӷ����ȱ�� |
| D����ɽ�����еķ������ʹ��������ݱ���ɴಢ�����䣬�Զ������Σ�� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com