
��֪��HCN�ĽṹΪH��C��N��
����о���������ȷ���ˮ�ⷢ�ͺ���Ƶÿ�ȩ ������Ҫ�Ļ���ԭ�ϣ�����һ�������¿ɷ������±仯��
��д����Ӧ���ͣ��� �������� ���� �������� ��
��д����Ӧ�۵Ļ�ѧ����ʽ�� �������� ��
д����֤����ȩ�к���ȩ����һ����ѧ��Ӧ����ʽ��
���������� ��
����EΪ��״���������ṹ��ʽ ��
����EΪ�߾������ṹ��ʽ ��
(5)����о��������ˮ��IJ���֮һJ���ڽ�ĸ���������²�����һ����������Q����һ���л���W����д��Q�ĵ���ʽ����������������������������д��ʵ������W��ȡһ����ʹ������Ȼ�̼��Һ��ɫ������P�Ļ�ѧ����ʽ�����������������������������۽�������ͭ˿�ھƾ��Ƶ���������Ƭ�̺��ٻ����������棬�۲쵽ͭ˿�ֱ�ù����Ͼ�����������������֮������������������������������������������������
 ѧ�ڸ�ϰһ��ͨѧϰ�ܶ�Ա��ĩ������ӱ����������ϵ�д�
ѧ�ڸ�ϰһ��ͨѧϰ�ܶ�Ա��ĩ������ӱ����������ϵ�д� â���̸����������������ϵ�д�
â���̸����������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| H2O |
 ��������Ҫ�Ļ���ԭ�ϣ�����һ�������¿ɷ������±仯��
��������Ҫ�Ļ���ԭ�ϣ�����һ�������¿ɷ������±仯��


 ��
��
 ��
��




�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| H2O(H+) |
 ��������Ҫ�Ļ���ԭ�ϣ�����һ�������¿ɷ������±仯��
��������Ҫ�Ļ���ԭ�ϣ�����һ�������¿ɷ������±仯��







| ŨH2SO4 |
| �� |
| ŨH2SO4 |
| �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| H2O |
 ��������Ҫ�Ļ���ԭ�ϣ�����һ�������¿ɷ������±仯��
��������Ҫ�Ļ���ԭ�ϣ�����һ�������¿ɷ������±仯��










| Ũ���� |
| 170�� |
| Ũ���� |
| 170�� |
| �� |
| �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪��HCN�ĽṹΪH-C��N��R-CN![]() RCOOH��R-Cl+CN���� R-CN+Cl����RΪ������������о���������ȷ���ˮ�ⷢ�ͺ���Ƶÿ�ȩ
RCOOH��R-Cl+CN���� R-CN+Cl����RΪ������������о���������ȷ���ˮ�ⷢ�ͺ���Ƶÿ�ȩ ![]() ��������Ҫ�Ļ���ԭ�ϣ�����һ�������¿ɷ������±仯��
��������Ҫ�Ļ���ԭ�ϣ�����һ�������¿ɷ������±仯��

��1��д����Ӧ���ͣ��� ���������� ���� �������� ��
��2��д����Ӧ�۵Ļ�ѧ����ʽ�� ��
д����֤����ȩ�к���ȩ����һ����ѧ��Ӧ����ʽ
��
��3����EΪ��״���������ṹ��ʽ ��
��4����EΪ�߾������ṹ��ʽ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ���㽭ʡ��У2009-2010ѧ��ȸ�����һ����������ѧ������ ���ͣ������
��֪��HCN�ĽṹΪH-C��N��R-CN RCOOH��R-Cl+CN���� R-CN+Cl����RΪ������������о���������ȷ���ˮ�ⷢ�ͺ���Ƶÿ�ȩ
RCOOH��R-Cl+CN���� R-CN+Cl����RΪ������������о���������ȷ���ˮ�ⷢ�ͺ���Ƶÿ�ȩ  ��������Ҫ�Ļ���ԭ�ϣ�����һ�������¿ɷ������±仯����12�֣�
��������Ҫ�Ļ���ԭ�ϣ�����һ�������¿ɷ������±仯����12�֣�
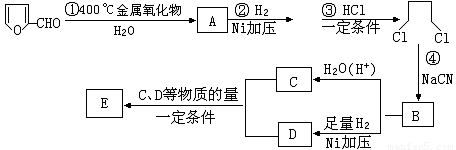
��1��д����Ӧ���ͣ��� ���������� ���� �������� ��
��2��д����Ӧ�۵Ļ�ѧ����ʽ�� ��
д����֤����ȩ�к���ȩ����һ����ѧ��Ӧ����ʽ
��
��3����EΪ��״���������ṹ��ʽ ��
��4����EΪ�߾������ṹ��ʽ ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com