��2012?�Ͼ�ģ�⣩�����ܡ�����δ�������������Դ��
��1��ʵ���ã�1g H
2��g��ȼ������Һ̬ˮʱ�ų�142.9kJ������������ȼ�յ��Ȼ�ѧ����ʽΪ
H2��g��+1/2O2��g��=H2O��l����H=-285.8kJ/mol
H2��g��+1/2O2��g��=H2O��l����H=-285.8kJ/mol
��
��2��ij��ѧ�Ҹ��ݡ�ԭ�Ӿ��á���˼�룬����������Ʊ�H
2�ķ�Ӧ����
��CaBr
2+H
2O
CaO+2HBr ��2HBr+Hg
HgBr
2+H
2��HgBr
2+
CaO
CaO
HgO
HgO
+
CaBr2
CaBr2
��2HgO
2Hg+O
2��
������ݡ�ԭ�Ӿ��á���˼�������������۵Ļ�ѧ����ʽ
��
���ݡ���ɫ��ѧ����˼�������÷�����H
2����Ҫȱ��
ѭ��������Ҫ�ܸߵ���������ʹ���ؽ�������������Ⱦ
ѭ��������Ҫ�ܸߵ���������ʹ���ؽ�������������Ⱦ
��
��3������ͨ��������ˮú���ķ����Ƶã����У�CO��g��+H
2O��g��?CO
2��g��+H
2��g����H��0����850��ʱ��K=1��
���������¶ȵ�950��ʱ���ﵽƽ��ʱK
��
��
1������ڡ�����С�ڡ����ڡ�����
��850��ʱ������һ�ݻ��ɱ���ܱ�������ͬʱ���� 1.0mol CO��3.0mol H
2O��1.0mol CO
2 ��x mol H
2����Ҫʹ������Ӧ��ʼʱ������Ӧ������У���xӦ�����������
0��x��3
0��x��3
��
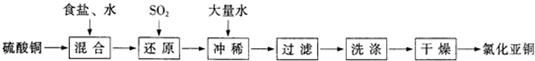
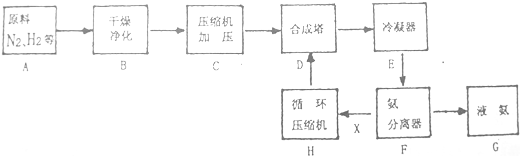
��4����ҵ�����У���������������Һ�����ŷŷ����еĶ������������ղ����⣬���Բ���������
��������ʣ�װ����ͼ1��ʾ���õ������������ĵ缫��ӦʽΪ
HSO3-+H2O-2e-=SO42-+3H+
HSO3-+H2O-2e-=SO42-+3H+
��
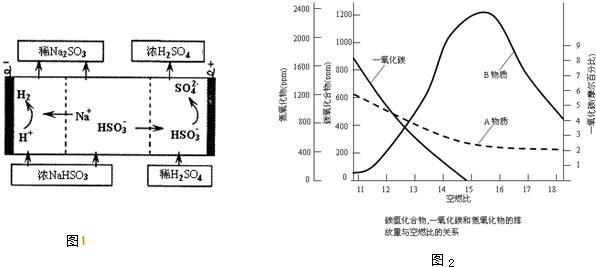
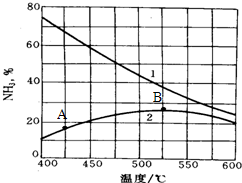
��5��һ����̼�����������̼�⻯����������β������Ҫ�ɷ֣���֪��ȼ�ȣ�������ȼ�����֮�ȣ���β���и��ɷ��ŷ�����ϵ��ͼ2��ʾ��B���ʵ�������
��������
��������
��


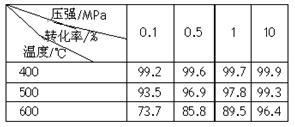
 ������ҵ��ͬ����ϰ��ϵ�д�
������ҵ��ͬ����ϰ��ϵ�д� С��ſ�ʱ��ҵϵ�д�
С��ſ�ʱ��ҵϵ�д�