
(1)����п�ǽ���п��(��Ҫ�ɷ���ZnS)ͨ����ѡ����ʹ��ת��Ϊ����п���ٰ�����п�ͽ�̿��ϣ��ڹķ�¯�м��ȵ�1 100��1 300 �棬ʹп���������
��д������п����Ҫ��Ӧ��
���շ�Ӧ��_________________________________________��
�ķ�¯�п��ܷ����ķ�Ӧ��___________________(��дһ��)
�ڴӱ��������ͳ������ԭ�ϽǶȿ���δ��������ò�����������_____________________________________________________________________________��
(2)��ҵ��ұ�������ǵ��������
��ұ�����ĵ����е��������������Ͼ���ʯ�����ƺ�ú�ĸ����Ʒ________(����������)��
�����������۵�ܸߣ�������ұ����Ҫ�������ʯ(Na3AlF6)����������________________________________________________��
�۹�ҵ��ұ����ʱ�õ�ԭ����Al2O3��������AlCl3����ԭ����
______________________________________________________��
(3)��ҵ�ϡ������Ƽ������Ҫ��Ӧ�Ļ�ѧ����ʽ��_______________________________________��
���е�CO2��Դ��______________��
(4)̼������Ʋ�����ԭ��֮һ����ҵ���Ʋ������ڲ�����¯�н��У����з�Ӧ֮һΪCaCO3��SiO2CaSiO3��CO2�����������������£���1 000a g CaCO3��60a g SiO2��ϣ������ɵ�CO2�ڱ�״���µ����Ϊ________________(�ú�a�Ĵ���ʽ��ʾ)��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij������ˮ�к�����̬�ȣ�ͨ������ʵ��ⶨ��Ũ�ȡ���ȡˮ��10.0ml����ƿ�У�����10.0ml��K I��Һ(����)�������ķ�ӦΪ��Cl2+2KI��2KCl+I2������ָʾ��2~3�Ρ���ȡһ�ζ�������������ˮ������ˮϴ��������ע��0.01mol��L-1Na2S2O3��Һ������Һ�棬���¶������۽���ƿ���ڵζ����½��еζ��������ķ�ӦΪ��I2+2Na2S2O3=2NaI+ 2Na2S4O6 �Իش������ʴ�
�Ų���ټ����ָʾ���� ��
�Ʋ����Ӧʹ�� ʽ�ζ��ܡ�
���жϴﵽ�ζ��յ��ʵ�������� ��
���ȵ�ʵ��Ũ�ȱ�����Ũ�Ȼ�ƫС���������ԭ����
��5����0.1032mol/L HCl��Һ�ζ�δ֪Ũ�ȵ�NaOH��Һ�����������ʵ������Ӱ�����
A����ʽ�ζ���δ�ñ�������Һ��ϴ B����ƿδ�ô���Һ��ϴ
C���ζ�ǰ�ζ��ܼ�������һ���ݣ��ζ���������ʧ��
D���ζ�ʱ����Һ������ƿ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����йػ�ѧ�����Լ���ѧ��������ı�������ȷ��һ����(����)
A. ��������(CH3COOOH)���ǻ�����(HOCH2COOH)������������ͬ������Ϊͬ���칹��
B. �ձ������˵�վй¶�ķ����Ժ��آ��Cs��ǰ�߱Ⱥ�����4������
C. ���������ƺ�̼�����Ƶĵ��뷽��ʽ����ʾΪNaHRO3⇋Na����HRO(R��C��S)
D. ������ĽṹʽΪHOCl����������ĵ���ʽΪH��[O O]2��H��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ȷ���ջ�ѧ������ѧ�û�ѧ�Ļ����������йر�����ȷ����
A��H��D��T��Ϊͬ��������
B���������еĻ�ѧ��Ϊ�Ǽ��Թ��ۼ�( )
C��NH4Cl�ĵ���ʽ��
D��S2���Ľṹʾ��ͼ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ϳɰ���ҵ�����Ṥҵ���������� ������ͼ��ʾ ��
��
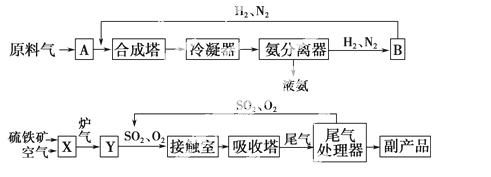
�ϳ����ͽӴ����еķ�Ӧ�ֱ�Ϊ
N2(g)��3H2(g) 2NH3(g)����H��0��
2SO2(g)��O2(g) 2SO3(g)����H��0��
(1)д���������豸�����ƣ�B________��X________��
(2)����ϳ����ͽӴ����е����嶼Ҫ�����ȴ�������������ȴ���������___________________��
(3)����ѭ�����������ԭ�ϵ������ʣ����������У�����ѭ����������________(�����)��
�����Ṥҵ���ںϳɰ���ҵ�������Ṥҵ
(4)��ҵ�ϳ���98.3%��Ũ��������SO3������ϡ�����ˮ��ԭ����_______________��
(5)��ҵ�����г��ð����ᷨ����β�������Դﵽ�� ����Ⱦ��
����Ⱦ�� �������õ�Ŀ�ġ����Ṥҵβ���е�SO2���������Եõ�һ�ֻ��ʣ��÷��ϵĻ�ѧʽ��___________________��
�������õ�Ŀ�ġ����Ṥҵβ���е�SO2���������Եõ�һ�ֻ��ʣ��÷��ϵĻ�ѧʽ��___________________��
(6)���ݻ�ѧ ƽ���ƶ�ԭ���������������ʩ��������________(�����)��
ƽ���ƶ�ԭ���������������ʩ��������________(�����)��
�ٺϳɰ���ҵ�ڸ�ѹ�½���
�ںϳɰ���ҵ�����Ṥҵ��ʹ�ô���
�ۼ�ʱ����Һ��������
�����Ṥҵ�У��������¯����Ҫ�й�������
�ݺϳɰ���ҵ�����Ṥҵ���������˵��¶�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ʯ��Ҫ������ơ�Ca3(PO4)2·H2O������ʯ��Ca5(OH)(PO4)3������ʽ���ڡ�ͼ(a)ΪĿǰ��������ʯ���õĴ������������ʪ��������ָ��ʯ�ù�������ֽ��Ʊ����ᡣͼ(b)���ȷ�������������������ʯ�Ƶ��������̡�
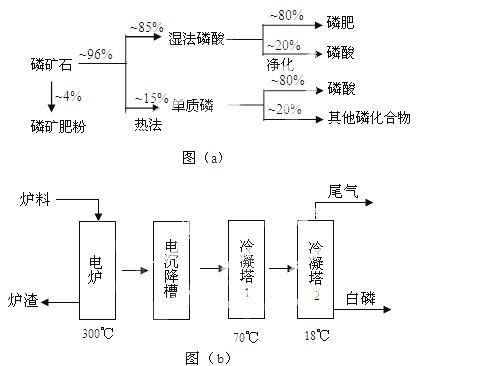
�������ʵ�����������£�
| �۵�/�� | �е�/�� | ��ע | |
| ���� | 44 | 280.5 | |
| PH3 | -133.8 | -87.8 | ������ˮ�����л�ԭ�� |
| SiF4 | -90 | -86 | ��ˮ�� |
�ش��������⣺
��1����������ʯ����Ҫ����;�����������ϣ�Լռ��ʯʹ������ ℅��
��2������ʯΪԭ�ϣ�ʪ�����������Ca5F(PO4)3��Ӧ�Ļ�ѧ����ʽΪ��  ������1���ۺϺ�������������Լ30%����ʯ�������Ƶ�85℅����Ʒ
������1���ۺϺ�������������Լ30%����ʯ�������Ƶ�85℅����Ʒ ���� �֡�
���� �֡�
��3����ͼ(b)��ʾ���ȷ���������ĵ�һ���ǽ��������衢������̿����ʯ��ϣ����·�Ӧ���ɰ��ס�¯������Ҫ�ɷ��ǣ� (�ѧʽ)������1����Ҫ�������ǣ� ������2����Ҫ�������ǣ�
��4��β������Ҫ���� ������������PH3��H2S��HF�ȣ���β ����ͨ�봿����Һ��
����ͨ�봿����Һ�� �ɳ�ȥ
�ɳ�ȥ 
��ͨ�����������Һ���ɳ�ȥ (���ѧʽ)
��5�������ʪ�����ᣬ�ȷ����Ṥ�ո��ӣ��ܺĸߣ����ŵ��ǣ�  ��
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���绷�����˽���ȫ���ֹʹ��������������ˮ����������������ø�Ч����ɫ���������������ȡ�����������һ�ּ��ױ�ը��ǿ���������壬������ˮ�����ȶ����ʻ���ɫ����������ʹ��ʱ���뾡����ϡ���������ϡ�ͣ�ͬʱ��Ҫ������ա�����ȡ�ʵ�����Ե�ⷨ�Ʊ�ClO2���������£�
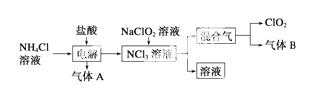
(1)ClO2������ԭ��________(��ǡ����ǡ�)������8���ӽṹ����ͼ��ʾ��ⷨ�ƵõIJ�������������B��ʹʯ����Һ����ɫ����ȥ���������ѡ��________��
A������ʳ��ˮ B����ʯ��
C��Ũ���� D������ˮ
(2)�ȶ��Զ���������Ϊ�ƹ�������ȶ����������Ͳ�Ʒ������˵����ȷ����________��
A���������ȿɹ㷺���ڹ�ҵ������ˮ����
B��Ӧ����ʳƷ��ҵ������Ч���ӳ�ʳƷ������
C���ȶ��Զ������ȵij��ִ�������˶������ȵ�ʹ�÷�Χ
D���ڹ������ͳ�Ʒ�������ڣ�Ҫ��ͨ��װ�úͼ�⼰����װ��
(3)ŷ������Ҫ��������������Ũ�����Ʊ�����ѧ��Ӧ����ʽΪ________________________________________________________________��
ȱ����Ҫ�Dz��ʵ͡���Ʒ���Է��룬��������Ⱦ������
(4)�ҹ��㷺���þ��������ϡ�͵������������������(NaClO2)��Ӧ�Ʊ�����ѧ����ʽ��________________________________________________________________________
________________________________________________________________________��
�˷����ŷ�������ŵ���______________________________________________��
(5)��ѧ�����о�����һ���µ��Ʊ����������������ữ�IJ���(H2C2O4)��Һ��ԭ�����ƣ���ѧ��Ӧ����ʽΪ_________________________________________________________
________________________________________________________________________��
�˷���������������桢����İ�ȫ�ԣ�ԭ����________________________________
________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
п��һ��Ӧ�ù㷺�Ľ�����Ŀǰ��ҵ����Ҫ���á�ʪ��������ұ��п��ij��п�����Ҫ�ɷ�ΪZnS����������FeS�������ɷ֣�������Ϊԭ��ұ��п�Ĺ���������ͼ��ʾ��
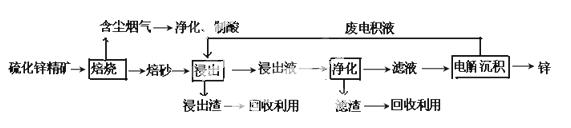
�ش��������⣺
��1����п����ı������������յķ���¯�н��У���������ɰ����Ҫ�ɷֵĻ�ѧʽΪ____��
��2�����չ����в����ĺ��������ɾ������ᣬ��������ں�����_______����.
��3������Һ�������������м������Ҫ����Ϊ________����������__________________��
��4�������������е������������壬��������Pb-Ag�Ͻ���Ե缫�������ݳ���������____��
��5���Ľ���пұ�����գ������ˡ���ѹ�������ȫʪ�����̣���ʡ�������¿�����Ⱦ�ı��չ��̣��ֿɻ��һ���й�ҵ��ֵ�ķǽ������ʡ�����ѹ������з�������Ҫ��Ӧ�����ӷ���ʽΪ___________________��
��6���ҹ��Ŵ������á�������ұ��п��������Ӧ�����ġ��칤������й��� ��������Ǧ���ļ��أ���¯��ʯʮ�װ����һ����ڣ�������Ȼ�������ú ̿����ʢ�������н�������Ѻ죬������������ٹ�ȡ������������
̿����ʢ�������н�������Ѻ죬������������ٹ�ȡ������������ ��ǦҲ��������п���չ�����Ҫ��Ӧ�Ļ�ѧ����ʽΪ____����ע��¯
��ǦҲ��������п���չ�����Ҫ��Ӧ�Ļ�ѧ����ʽΪ____����ע��¯ ��ʯ����Ҫ�ɷ�Ϊ̼��п����Ǧ��ָ����п��
��ʯ����Ҫ�ɷ�Ϊ̼��п����Ǧ��ָ����п��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ԭ���������������A��B��C��D��E����Ԫ�أ�A�Ƕ������������������������ķǽ���Ԫ�أ�BԪ�ص�ԭ�ӼȲ���ʧȥҲ���õ����ӣ����̬ԭ��ÿ�ֹ���е�������ͬ��CԪ�صļ۵��ӹ���Ϊnsnnpn��1��D����������������Ӳ���֮��Ϊ3��1��E�ǵؿ��к������������Ľ���Ԫ�أ���Ͻ���;��㣬�������
(1)B��D�γɵķǼ��Է���������ԭ�ӵŶԵ�������________������ԭ�ӵ��ӻ��������Ϊ________��
(2)A�ֱ���B��C��D���γɵ�����Ϊ10�Ļ�������ǵķе��ɸߵ��͵�˳����____________(д����ʽ)�����ǵ��ȶ���������ǿ��˳����_______________________________________________________(д����ʽ)��
(3)����ABC��BA2D�Ŀռ乹�ͷֱ���________��________��
(4)B��C��D����Ԫ�صĵ縺���ɴ�С��˳����_______ _(��Ԫ�ط��ű�ʾ)����һ�������ɴ�С��˳����________(��Ԫ�ط��ű�ʾ)��
_(��Ԫ�ط��ű�ʾ)����һ�������ɴ�С��˳����________(��Ԫ�ط��ű�ʾ)��
(5)C�ĵ��ʷ����д���________���м���________���Ҽ���
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com