
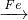 FeCl3
FeCl3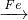 FeCl2��HNO3
FeCl2��HNO3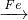 Fe��NO3��3
Fe��NO3��3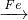 Fe��NO3��2���ʴ�Ϊ��FeCl3 ��Fe��NO3��3��
Fe��NO3��2���ʴ�Ϊ��FeCl3 ��Fe��NO3��3��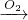 N2
N2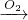 ��NO����AΪ NH3������ʽΪ
��NO����AΪ NH3������ʽΪ ��BΪN2���ṹʽΪN��N���ʴ�Ϊ��
��BΪN2���ṹʽΪN��N���ʴ�Ϊ�� ��N��N��
��N��N�� Al��OH��3
Al��OH��3 AlO2-����Ӧ�������ӷ���ʽΪ
AlO2-����Ӧ�������ӷ���ʽΪ Na2CO3
Na2CO3 NaHCO3��25��ʱ��pH��Ϊ10��NaOH��Na2CO3����Һ�У���ˮ�����������������Ũ�ȷֱ�Ϊ��NaOH��c��OH-��=10-10mol/L��Na2CO3��c��OH-��=10-4mol/L��������ˮ�����������������Ũ��֮��Ϊ10-6��1��1��106���ʴ�Ϊ��10-6��1��1��106��
NaHCO3��25��ʱ��pH��Ϊ10��NaOH��Na2CO3����Һ�У���ˮ�����������������Ũ�ȷֱ�Ϊ��NaOH��c��OH-��=10-10mol/L��Na2CO3��c��OH-��=10-4mol/L��������ˮ�����������������Ũ��֮��Ϊ10-6��1��1��106���ʴ�Ϊ��10-6��1��1��106��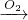 N2
N2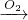 ��NO����AΪ NH3���Դ���д����ʽ�ͽṹʽ��
��NO����AΪ NH3���Դ���д����ʽ�ͽṹʽ�� Al��OH��3
Al��OH��3 AlO2-����Ӧ�������ӷ���ʽΪ
AlO2-����Ӧ�������ӷ���ʽΪ Na2CO3
Na2CO3 NaHCO3��25��ʱ��pH��Ϊ10��NaOH��Na2CO3����Һ�У���ˮ�����������������Ũ�ȷֱ�Ϊ��NaOH��c��OH-��=10-10mol/L��Na2CO3��c��OH-��=10-4mol/L���Դ˼�������Ũ�ȹ�ϵ��
NaHCO3��25��ʱ��pH��Ϊ10��NaOH��Na2CO3����Һ�У���ˮ�����������������Ũ�ȷֱ�Ϊ��NaOH��c��OH-��=10-10mol/L��Na2CO3��c��OH-��=10-4mol/L���Դ˼�������Ũ�ȹ�ϵ��

 �����������Ů��ͯ������ϵ�д�
�����������Ů��ͯ������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
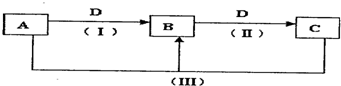
 A��B��C��D������ѧ��ѧ�г������ʣ�����A��B��C������ͬһ��Ԫ�أ���һ���������ת����ϵ��ͼ��ʾ�����ַ�Ӧ�е�ˮ����ȥ����
A��B��C��D������ѧ��ѧ�г������ʣ�����A��B��C������ͬһ��Ԫ�أ���һ���������ת����ϵ��ͼ��ʾ�����ַ�Ӧ�е�ˮ����ȥ���� Fe��OH��3+3H+
Fe��OH��3+3H+ Fe��OH��3+3H+
Fe��OH��3+3H+�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
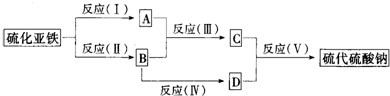
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com