
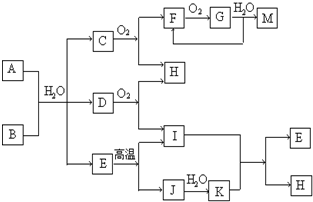
��֪��AΪ��ʽ�Σ�BΪij�����������Ԫ�صĻ��������B�Ļ�ѧʽ�й���32�����ӣ����³�ѹ��C��D��F��G��I������̬����G�ʺ���ɫ�����������Ϊ��ɫ�������ʵ���A��B��������ˮ��ֻ����ǡ����ȫ��Ӧ��ͼ�з�Ӧ�������������⣩������ȥ��
��1��д��B�ĵ���ʽ
��2��д�����з�Ӧ�����ӷ���ʽ��
����A��Һ�м���M
����A��Һ�м������NaOH��Һ��������
��3��C�����ڴ����а���ȼ�գ�����ΪH��һ�ֵ��ʣ�д���÷�Ӧ�Ļ�ѧ����ʽ��
��4��д��A��B��Ӧ�Ļ�ѧ����ʽ ��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��֪��AΪ��ʽ�Σ�BΪij�����������Ԫ�صĻ��������B�Ļ�ѧʽ�й���36�����ӣ����³�ѹ��C��D��F��G��I������̬����G�ʺ���ɫ�����������Ϊ��ɫ��H�ڳ���������ɫҺ�壮�����ʵ���A��B��������H��ֻ����ǡ����ȫ��Ӧ��ͼ�з�Ӧ�������������⣩������ȥ��
��֪��AΪ��ʽ�Σ�BΪij�����������Ԫ�صĻ��������B�Ļ�ѧʽ�й���36�����ӣ����³�ѹ��C��D��F��G��I������̬����G�ʺ���ɫ�����������Ϊ��ɫ��H�ڳ���������ɫҺ�壮�����ʵ���A��B��������H��ֻ����ǡ����ȫ��Ӧ��ͼ�з�Ӧ�������������⣩������ȥ��

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪��AΪ��ʽ�Σ�BΪij�����������Ԫ�صĻ��������B�Ļ�ѧʽ�й���32�����ӣ� ���³�ѹ��C��D��F��G��I������̬ ����G�ʺ���ɫ�����������Ϊ��ɫ�������ʵ���A��B��������ˮ��ֻ����ǡ����ȫ��Ӧ��ͼ�з�Ӧ�������������⣩������ȥ��

��2��д��A��B��Ӧ�Ļ�ѧ����ʽ ��
��3��д�����з�Ӧ�����ӷ���ʽ��
����A��Һ�м���M
����A��Һ�м������NaOH��Һ��������
��4��C�����ڴ����а���ȼ�գ�����ΪH��һ�ֵ��ʣ�д���÷�Ӧ�Ļ�ѧ����ʽ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010-2011ѧ�������и���12���¿������ۣ���ѧ���� ���ͣ������
��֪��AΪ��ʽ�Σ�BΪij�����������Ԫ�صĻ��������B�Ļ�ѧʽ�й���32�����ӣ� ���³�ѹ��C��D��F��G��I������̬ ����G�ʺ���ɫ�����������Ϊ��ɫ�������ʵ���A��B��������ˮ��ֻ����ǡ����ȫ��Ӧ��ͼ�з�Ӧ�������������⣩������ȥ��

��1��д��B�ĵ���ʽ
��2��д�����з�Ӧ�����ӷ���ʽ��
����A��Һ�м���M
����A��Һ�м������NaOH��Һ��������
��3��C�����ڴ����а���ȼ�գ�����ΪH��һ�ֵ��ʣ�д���÷�Ӧ�Ļ�ѧ����ʽ��
��4��д��A��B��Ӧ�Ļ�ѧ����ʽ ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com