
����Ŀ���������ֳƼ��ѣ����DME���۵�Ϊ-141.5�棬�е�Ϊ-24.9�档��������Һ��ʯ����(LPG)���ƣ�����Ϊ��21���͵����ȼ�ϡ����ɺϳ���(CO��H2)�Ʊ������ѵķ�Ӧԭ�����£�
��CO(g)+2H2(g)![]() CH3OH(g) ��H1= -90.0 kJ��mol-1
CH3OH(g) ��H1= -90.0 kJ��mol-1
��2CH3OH(g)![]() CH3OCH3(g)+H2O(g) ��H2=-20.0kJ��mol-l
CH3OCH3(g)+H2O(g) ��H2=-20.0kJ��mol-l
�ش��������⣺
��1����Ӧ��Ϊ��___________(����ӡ����١�)�ķ�Ӧ��__________ (����¡����¡�)�����Է����С�
��2��д���ɺϳ���(CO��H2) ֱ���Ʊ������ѵ��Ȼ�ѧ����ʽ��__________________________________��
��3���¶�Ϊ500Kʱ����2L���ܱ������г���2 mol CO��6 molH2������Ӧ�١��ڣ�5 minʱ�ﵽƽ�⣬ƽ��ʱCO��ת����Ϊ60%��c(CH3OCH3)=0.2 mol��L-1����H2��ʾ��Ӧ�ٵ�������______________����Ӧ�ڵ�ƽ�ⳣ��K=________________��
����500Kʱ�����������n(CH3OCH3)=2n (CH3OH)����ʱ��Ӧ�ڵ�v��__ v��(����>���� ��< ������=��)��
��4���о����֣��������ͬ�������м������ʵ�����ͬ��CO��H2������Ӧ�١��ڣ��ڲ�ͬ�¶Ⱥ�����������¾�����ͬ��Ӧʱ��������ʵ�����ݣ�
T(K) | ���� | COת����(%) | CH3OCH3ѡ����(%) |
473 | �� | 10 | 36 |
500 | �� | 12 | 39 |
500 | Cu/ZnO | 20 | 81 |
[��ע]������ѡ���ԣ�ת����CO������CH3OCH3�İٷֱ�
����ͬ�¶��£�ѡ��Cu/ZnO���������ô�����____________ (����)��
A.�ٽ�ƽ�������ƶ� B.��߷�Ӧ���� C.���ͷ�Ӧ�Ļ��
D.�ı䷴Ӧ���ʱ� E.���CO��ƽ��ת����
�ڱ���ʵ�����ݱ�������500Kʱ������Cu/ZnO��COת����CH3OCH3��ѡ������������Ӱ�죬��ԭ����_________________________________________________��
���𰸡� ��С ���� 2CO(g)+4H2(g)![]() CH3OCH3(g)+H2O(g) ��H= -200 kJ��mol-l 0.24mol/(L��min) 1 < B��C �ɱ������ݿ�֪����ʱ��Ӧ��ϵ��δ����ƽ�⣬�����ɼ��ٷ�Ӧ�ڣ��Ӷ�����ͬʱ�������ɸ����CH3OCH3
CH3OCH3(g)+H2O(g) ��H= -200 kJ��mol-l 0.24mol/(L��min) 1 < B��C �ɱ������ݿ�֪����ʱ��Ӧ��ϵ��δ����ƽ�⣬�����ɼ��ٷ�Ӧ�ڣ��Ӷ�����ͬʱ�������ɸ����CH3OCH3
����������1����Ӧ��CO(g)+2H2(g)![]() CH3OH(g) ��H1= -90.0 kJ��mol-1 ������������٣����ؼ��٣�����S<0���÷�ӦΪ���ȷ�Ӧ��Ҫ���Է����У���H-T��S<0��T������ѧ�¶ȣ�����ֵ���ʵ���ʱ����H-T��S<0���÷�Ӧ�ڵ����������Է����У�
CH3OH(g) ��H1= -90.0 kJ��mol-1 ������������٣����ؼ��٣�����S<0���÷�ӦΪ���ȷ�Ӧ��Ҫ���Է����У���H-T��S<0��T������ѧ�¶ȣ�����ֵ���ʵ���ʱ����H-T��S<0���÷�Ӧ�ڵ����������Է����У�
��2���ɺϳ���(CO��H2) ֱ���Ʊ������ѵĻ�ѧ����ʽ��2CO(g)+4H2(g)![]() CH3OCH3(g)+H2O(g)���÷�Ӧ���ɢ١�2+�ڵ������ʸ÷�Ӧ�ġ�H=-200 kJ��mol-l���ʸ÷�Ӧ���Ȼ�ѧ����ʽΪ��2CO(g)+4H2(g)
CH3OCH3(g)+H2O(g)���÷�Ӧ���ɢ١�2+�ڵ������ʸ÷�Ӧ�ġ�H=-200 kJ��mol-l���ʸ÷�Ӧ���Ȼ�ѧ����ʽΪ��2CO(g)+4H2(g)![]() CH3OCH3(g)+H2O(g) ��H= -200 kJ��mol-l��
CH3OCH3(g)+H2O(g) ��H= -200 kJ��mol-l��
��3���¶�Ϊ500Kʱ����2L���ܱ������г���2 mol CO��6 molH2������Ӧ��������5 minʱ�ﵽƽ�⣬ƽ��ʱCO��ת����Ϊ60%��c(CH3OCH3)=0.2 mol��L-1��
��CO(g)+2H2(g)![]() CH3OH(g)
CH3OH(g)
��ʼŨ�� 1 3 0
��ӦŨ�� 0.6 1.2 0.6
ƽ��Ũ�� 0.4 1.8 0.6
H2��ʾ��Ӧ�ٵ�������v=![]() =0.24mol/(L��min)
=0.24mol/(L��min)
��2CH3OH(g)![]() CH3OCH3(g)+H2O(g)
CH3OCH3(g)+H2O(g)
��ʼŨ�� 0.6 0 0
��ӦŨ�� 0.4 0.2 0.2
ƽ��Ũ�� 0.2 0.2 0.2
��Ӧ�ڵ�ƽ�ⳣ��K= ![]() = 1��
= 1��
����500Kʱ�����������n(CH3OCH3)=2n (CH3OH)����ʱn(CH3OCH3)=n��H2O��=2n (CH3OH)��c(CH3OCH3)=c��H2O��=2c (CH3OH)����ʱQc=0.25>K��ƽ�⽫�����ƶ�����ʱ
��Ӧ�ڵ�v��< v����
��4�����ݱ������ͬ�¶��£�ѡ��Cu/ZnO������������ֻ�ܼӿ췴Ӧ���ʡ����ͷ�Ӧ��ܣ���ѡB��C��
�ڱ���ʵ�����ݱ�������500Kʱ������Cu/ZnO��COת����CH3OCH3��ѡ������������Ӱ�죬�ɱ������ݿ�֪����ʱ��Ӧ��ϵ��δ����ƽ�⣬�����ɼ��ٷ�Ӧ�ڣ��Ӷ�����ͬʱ�������ɸ����CH3OCH3��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ijͬѧ�����й�ͭ�����ᡢ���ữѧ���ʵ�ʵ�飬ʵ�������ͼ��ʾ��

�����۾��۲쵽ʵ��������Һ����ɫ���Թܿ��к���ɫ�������������˵����ȷ����
A. ���Թܿ��к���ɫ���������ԭ�������ᱻ��ԭΪNO2
B. �ɢ۵�����ɵó����ۣ�Cu����ϡ���ᷴӦ
C. ���з�Ӧ�����ӷ���ʽ��3Cu��2NO3-+8H+ === 3Cu2+��2NO����4H2O
D. ���п��ܲ���SO2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��(6��)Ϊ̽����ɫ����X(��������Ԫ��)����ɺ����ʣ���Ʋ��������ʵ�飺

��ش�
(1)X�Ļ�ѧʽ��________��
(2)�������ϡ���ᷴӦ�����ӷ���ʽ��___________��
(3)���������°���������X������һ�����嵥�ʣ�д���÷�Ӧ�Ļ�ѧ����ʽ__________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij��Һ�к���HCO3����CO32����SO32����Na����NO3���������ӡ��������м���������Ʒ�ĩ��ַ�Ӧ����Һ����仯���Բ��ƣ�����Һ������Ũ�ȱ��ֲ������
A��CO32����NO3�� B��NO3��
C��SO32����NO3�� D��CO32����NO3����Na��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������1molHNO3��1molH2OS4�Ļ����Һ��������������������Һ�н�������a��b���������۵����ʵ����Ĺ�ϵ��ͼ��ʾ(��֪ϡ����Ļ�ԭ����ֻ��NO)�������й��жϲ���ȷ����()

A. a��ʾn(Fe3+)�ı仯����
B. n1=0.75
C. P��ʱ��n(Fe2+)=0.5625 mol
D. ��P����Һ�м���ͭ�ۣ������ܽ�14.4g
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����һ��ɫ��Һ�����п��ܺ��� Fe3+��Al2+ ��Fe2+��Mg2+��Cu2+��NH4+��K+��CO32-��SO42-�����ӵļ��֣�Ϊ������ɷ֣�ȡ����Һ�ֱ�������ĸ�ʵ�飬��̽�����й���������ͼ��ʾ��

���������ͼ�ƶϣ�
(1)ԭ��Һ��һ�����ڵ���������(д��ѧʽ)��_________________________��
(2)д��ʵ����в����ij����У�______________________��
д����A��B��������������Ӧ�����ӷ���ʽ�� ______________________��
(3)ʵ����в�����ɫ��ζ�����������Ļ�ѧ����ʽΪ��______________________��
(4)ʵ����м���ϡ�����Ŀ����______________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ҩ�� W �Ըΰ������ƾ��кܺõ�Ч����һ�ֺϳ�ҩ�� W ��·�����£�

��֪������Ϣ��
��2HCHO +NaOH��CH3OH+ HCOONa
��![]()
�۶�ϩ�������ĽṹͲʽΪ![]()
�ش��������⣺
(1)�л��� A �к��еĹ�������̼̼˫����_____(������)��
(2)�ڢٲ���Ӧ�г�����![]() �⣬��������һ����˲���Ľṹ��ʽΪ_____��
�⣬��������һ����˲���Ľṹ��ʽΪ_____��
(3)H �Ľṹ��ʽΪ_____��
(4)�ڢݲ��ķ�Ӧ������_____��
(5)L ��ҩ�� W ��ͬ���칹�壬ͬʱ�������������� L �� _____��(�����������칹)��
�ٷ����к��б����������ϣ��� 4 ��ȡ����
�ڼ����� FeCl3����Һ������ɫ��Ӧ�������� NaHCO3����Һ��Ӧ��������
��1mol���� 3 mol NaOH ��ȫ��Ӧ�����к˴Ź�������Ϊ����壬�������Ϊ 1:2:2:3 �Ľṹ��ʽΪ ____��
(6)д����![]() �Ʊ���ϩ������
�Ʊ���ϩ������![]() �ĺϳ�·����_____�����Լ���ѡ)��
�ĺϳ�·����_____�����Լ���ѡ)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ҵ�Ϸ�������Ϊ���ȼ���е���������1886�귨����ѧ��H��M0issanͨ�������⻯��(KHF2)�ķ�������ˮ��Һ��һ���Ƶ÷�������֪��KF+HF=KHF2���Ʊ������ĵ��װ����ͼ��ʾ������˵���������
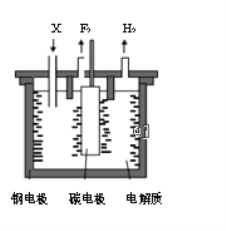
A. �ֵ缫���Դ�ĸ�������
B. �������費�ϲ����X��KF
C. �������������ұ������
D. ���⻯���ڷ������п��Ե���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ҵ����������(��Ҫ�ɷ�ΪA12O3����FeO����)Ϊԭ��ұ�����Ĺ�������������
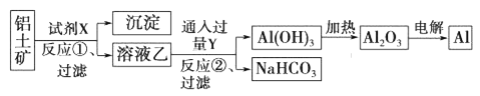
����˵����ȷ����
A. �Լ�X����������������Һ��Ҳ������������Һ
B. ��Ӧ�١����˺����ó���Ϊ��������
C. ͼ����ʾת����Ӧ������������ԭ��Ӧ
D. ��Ӧ�ڵĻ�ѧ����ʽΪNaAlO2+CO2+2H2O=A1(OH)3��+NaHCO3
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com