
�ټ��������Na2CO3��Һ
�ڼ��������NaOH��Һ
�ۼ��������BaCl2��Һ
�ܹ��˳�ȥ����
������Һ�м��������pH
��ͨ�������ӽ������þ�����ˮ
���г���˳����ȷ����( )
A.�٢ڢۢܢݢ� B.�ڢۢܢ٢ݢ�
C.�ڢܢݢޢۢ� D.�٢ۢܢڢݢ�

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
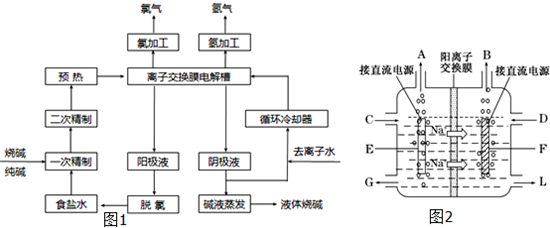
 ��ҵ�ϵ���Ƽ�ļ����������ӽ���Ĥ������Ҫԭ���DZ���ʳ��ˮ����ͼΪ���ӽ���Ĥ�����ԭ��ʾ��ͼ����ش��������⣺
��ҵ�ϵ���Ƽ�ļ����������ӽ���Ĥ������Ҫԭ���DZ���ʳ��ˮ����ͼΪ���ӽ���Ĥ�����ԭ��ʾ��ͼ����ش��������⣺
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��13�֣���ⷨ�Ƽ����Ҫԭ���DZ���ʳ��ˮ�����ڴ���ˮ�к�����ɳ��Ca2����Mg2����Fe3����SO�����ʣ������ϵ��Ҫ����˱��뾭�����ƣ�ijͬѧ�����һ���Ʊ����ε�ʵ�鷽�����������£����ڳ������Լ��Թ�������
����1��ȡһ�����Ĵ��Σ������ձ��У�����������ˮ����ɴ���ˮ��
����2�������ˮ�м�������Լ���Ȼ����й��ˣ���ȥ�����������Һ�м�������
������ˮ��pH��
����3�����õ�����Һ����Ũ������ȴ���ᾧ�����ˡ���ɼ��þ��Σ�
��ش��������⣺
��1������ʵ���еĹ��˲�����Ҫ�ձ���____________��____________�Ȳ���������
��2������2�г���Na2CO3��NaOH��BaCl2��Ϊ�����Լ������������Լ���˳��Ϊ��
________________��
��3������2�У��жϼ���BaCl2�ѹ����ķ����ǣ�_______________________________
_______________________��
��4������2�У��������������pH�ٹ��ˣ������ʵ��������Ӱ�죬��ԭ����
________________________________________________________________________��
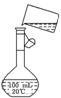 ��5��Ϊ���龫�δ��ȣ�������150 mL 0��2 mol/L NaCl�����Σ���Һ����ͼ�Ǹ�
��5��Ϊ���龫�δ��ȣ�������150 mL 0��2 mol/L NaCl�����Σ���Һ����ͼ�Ǹ�
ͬѧת����Һ��ʾ��ͼ��ͼ�е����������ǣ�
________________________________________________
________________________________________________��
���ڶ���ʱ���ӣ���������Һ��Ũ��_____________________________________
0��2 mol/L������ڡ���С�ڡ����������ݲ��������̶��ߣ�Ӧ���õĴ��������ǣ�________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�������ѧ�ڻ�ѧһ�ָ�ϰ����ʵ�鵽��ѧ����Ԫ�����Ծ���1�� ���ͣ�ʵ����
��13�֣���ⷨ�Ƽ����Ҫԭ���DZ���ʳ��ˮ�����ڴ���ˮ�к�����ɳ��Ca2����Mg2����Fe3����SO�����ʣ������ϵ��Ҫ����˱��뾭�����ƣ�ijͬѧ�����һ���Ʊ����ε�ʵ�鷽�����������£����ڳ������Լ��Թ�������
����1��ȡһ�����Ĵ��Σ������ձ��У�����������ˮ����ɴ���ˮ��
����2�������ˮ�м�������Լ���Ȼ����й��ˣ���ȥ�����������Һ�м�������
������ˮ��pH��
����3�����õ�����Һ����Ũ������ȴ���ᾧ�����ˡ���ɼ��þ��Σ�
��ش��������⣺
��1������ʵ���еĹ��˲�����Ҫ�ձ���____________��____________�Ȳ���������
��2������2�г���Na2CO3��NaOH��BaCl2��Ϊ�����Լ������������Լ���˳��Ϊ��
________________��
��3������2�У��жϼ���BaCl2�ѹ����ķ����ǣ�_______________________________
_______________________��
��4������2�У��������������pH�ٹ��ˣ������ʵ��������Ӱ�죬��ԭ����
________________________________________________________________________��
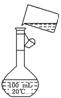 ��5��Ϊ���龫�δ��ȣ�������150 mL 0��2 mol/L NaCl�����Σ���Һ����ͼ�Ǹ�
��5��Ϊ���龫�δ��ȣ�������150 mL 0��2 mol/L NaCl�����Σ���Һ����ͼ�Ǹ�
ͬѧת����Һ��ʾ��ͼ��ͼ�е����������ǣ�[��Դ:ѧ|��|��]
________________________________________________
________________________________________________��
���ڶ���ʱ���ӣ���������Һ��Ũ��_____________________________________
0��2 mol/L������ڡ���С�ڡ����������ݲ��������̶��ߣ�Ӧ���õĴ��������ǣ� ________________________________________________________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com