ij��ѧ��ȤС��ⶨijFeCl
3��Ʒ��ֻ������FeCl
2���ʣ�����Ԫ�ص�����������������ʵ�鲽����в�����
�ٳ�ȡa g��Ʒ�������ձ��У�
�ڼ����������������ˮ��ʹ��Ʒ�ܽ⣬Ȼ��ȷ���Ƴ�250.00mL��Һ��
����ȡ25.00mL���������õ���Һ�������ձ��У�������������ˮ��ʹ��Ӧ��ȫ��
�ܼ��������ˮ����ֽ��裬ʹ������ȫ��
�ݹ��ˣ�ϴ�ӳ�����
������ת�Ƶ�ij�����ڣ����ȡ����裬ֱ�������ɺ��ɫȫ����Ϊ����ɫ���ڸ���������ȴ�����º�����
�ߡ���
����������������ش�
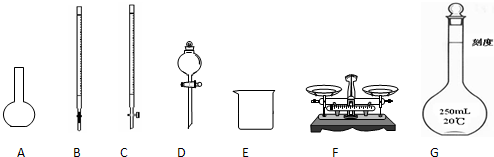
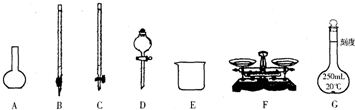
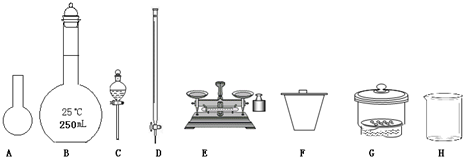
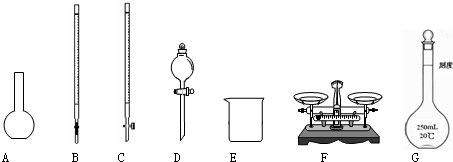
��1��ͼ��ʾ�����У���ʵ�鲽��٢ڢ��б����õ���������E��
CFG
CFG
������ĸ����
��2�������������������
��ǿ��Һ���ԣ���ֹFe2+Fe3+ˮ�⣻
��ǿ��Һ���ԣ���ֹFe2+Fe3+ˮ�⣻
��
��3��д��������з�����Ӧ�����ӷ���ʽ
Fe3++3NH3?H2O�TFe��OH��3��+3NH4+
Fe3++3NH3?H2O�TFe��OH��3��+3NH4+
��
��4����Ʒ�е�����Fe
2+�н�ǿ�Ļ�ԭ�ԣ���ɲ���ƽ���з�Ӧ�����ӷ���ʽ
6
6
Fe
2++
1
1
ClO
3-+
6
6
�T
6
6
Fe
3++
1
1
Cl
-+
3
3
H
2O
�������Ϸ���������ʵ����������ˮ��ΪNaClO
3ʱ����ʵ�������
��Ӱ��
��Ӱ��
����ƫ��ƫС��Ӱ�죩�������ʵ�����NaClO
3��Cl
2������Ч��֮��Ϊ
3��1
3��1
��5���ڢ��IJ����У���������ת�Ƶ�
����
����
�����������ƣ��м��ȣ���ȴ�����£�����������Ϊm
1g���ٴμ��Ȳ���ȴ�����³���������Ϊm
2g����m
1��m
2��ֵ�ϴ������IJ���Ӧ����
��������һ��ʱ������������ڸ���������ȴ���ٳ�������������γƵõ����������0.1gΪֹ
��������һ��ʱ������������ڸ���������ȴ���ٳ�������������γƵõ����������0.1gΪֹ
��
��6����ʵ����Ҫ���������ٴ�����
5
5
��������������W
1g�����������������������W
2g������Ʒ����Ԫ�ص���������Ϊ
�����г���ʽ�����軯��
��7����֪Cl
2���ȵ�NaOH��Һ�ɷ������·�Ӧ��
3Cl
2+6NaOH
5NaCl+NaClO
3+3H
2O
��Fe
3+��KClO
3��Cl
2������������������ǿ������˳����
KClO3��Cl2��Fe3+
KClO3��Cl2��Fe3+
��