
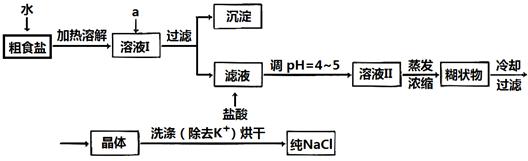
 ������ƽ��ҩ�ס����������___ _____�����������ƣ���
������ƽ��ҩ�ס����������___ _____�����������ƣ���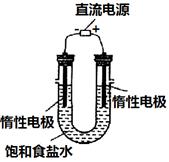
 MnCl2+Cl2��+2H2O���ݴˣ���������������װ����ѡ���Ʊ����ռ�H2��װ��_______(�����)���Ʊ����ռ��������Cl2��װ��_________������ţ���
MnCl2+Cl2��+2H2O���ݴˣ���������������װ����ѡ���Ʊ����ռ�H2��װ��_______(�����)���Ʊ����ռ��������Cl2��װ��_________������ţ���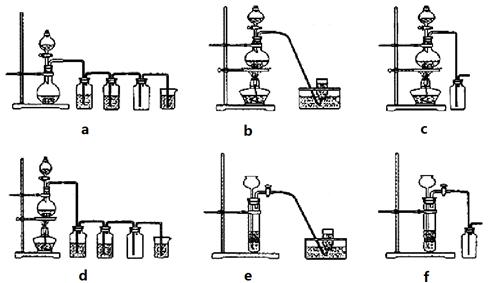
 ����ѧҵ���Ե�����ϵ�д�
����ѧҵ���Ե�����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
| | ѡ���Լ� | ʵ������ |
| ����1 | | |
| ����2 | | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
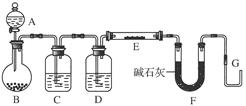
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
 ƷԤ�������õ����в��ἰ�����ε���Һ��
ƷԤ�������õ����в��ἰ�����ε���Һ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
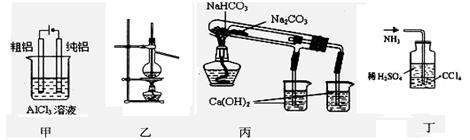
| A���ü�ͼװ�õ�⾫���� | B������ͼװ�ÿ�����ʯ�͵ķ��� |
| C����ͼװ�ÿ���֤NaHCO3��Na2CO3���ȶ��� | D����ͼװ�ÿ����պ��а�����β�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A������ƿ��ԭ����������ˮ | B������ʱ���ӿ̶��� |
| C��������NaOH��Һ�������ձ��� | D������ʱ�����Ϊ��������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��п����ϡHNO3��Ӧ�Ʊ�H2 |
| B��������ڵ�Al2O3�Ʊ�O2 |
| C������MnO2��ŨHCl��Ӧ�Ʊ�Cl2 |
| D������ʯ��ŨH2SO4��Ӧ�Ʊ�CO2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
| A��KI��Һ | B��������Һ | C��NaOH��Һ | D��ϡH2SO4E����ˮ |
 ɫ��������Һ��ɫ�ı仯���ش��������⣺
ɫ��������Һ��ɫ�ı仯���ش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
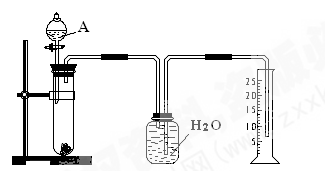
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com