

���� A����1��±�����ڼ�Ĵ���Һ���ȣ�������ȥ��Ӧ��
��2���Ҵ��е�ͣ��ӷ�����ϩ����ˮ�е��巢���ӳɷ�Ӧ���Ҵ�����ˮ����Ӧ��
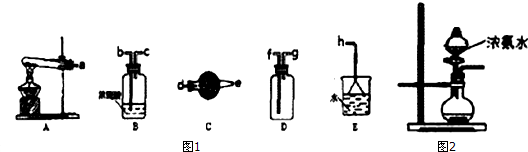
B����1�����ֲ��������ʱ��Ӧ�����Ⱥ�˳�������ȼ����������Ȼ���ȥ���������ټ������������ϩ��
��2�����ݶ���������Ư��Ʒ������������Ĵ��ڣ�
��3��װ�â���ȷ����������װ�â������������Ը��������Һ��ɫ֤����������к�����ϩ��
��4��ʵ�����Ʊ���ϩ���õ�ԭ��Ϊ�Ҵ���Ũ��������������ˮ������Ӧ�����Ǽ��ȵ�170�棬�ݴ�д����Ӧ�Ļ�ѧ����ʽ��
��� �⣺A����1���������һ����������ȥһ���廯����ӣ��������е�̼̼������Ϊ˫������Ӧ�ķ���ʽΪ��CH3CH2Br+NaOH$��_{��}^{�Ҵ�}$CH2=CH2��+NaBr+H2O��
�ʴ�Ϊ��CH3CH2Br+NaOH$��_{��}^{�Ҵ�}$CH2=CH2��+NaBr+H2O��
��2���Ҵ��е�ͣ��ӷ������Ҵ���ˮ��������Ȼ��ܣ���ϩ����ˮ�е��巢���ӳɷ�Ӧ����ˮ��ɫ���Ҵ�����ˮ����Ӧ���ɼ�����ϩ�Ĵ��ڣ�
�ʴ�Ϊ����ȥ�ӷ��������Ҵ�����ˮ��������Ȼ�̼��Һ��
B����1���������������Ʒ����Һ��������ϩ�ø������������Һ����ϩ�Ͷ���������ʹ�������������Һ��ɫ�������ȼ����������Ȼ�������ϩ��ͬ�ڼ�����ϩ֮ǰ��NaOH��Һ����SO2����ͨ��Ʒ����Һ����ɫȷ��SO2�ѳ��ɾ�������ø������������Һ��ɫ������ϩ����װ��I��������SO2���Թ���Ʒ����Һ��ɫ��˵������SO2��װ��II�Թ�װ��NaOH��Һ��ȥSO2��װ��III�Թ�ͨ��Ʒ����Һ����ɫȷ��SO2�ѳ��ɾ���װ��IV ͨ���������������Һ��ɫ������ϩ��
�ʴ�Ϊ��C��D��C��B��
��2���Ҵ���Ũ����Ĵ������·�����������ˮ��ȡ��ϩ���Ҵ���������ȥ��Ӧ����Ӧ�Ļ�ѧ����ʽΪ��CH3CH2OH$��_{170��}^{Ũ����}$CH2=CH2��+H2O��
�ʴ�Ϊ��CH3CH2OH$��_{170��}^{Ũ����}$CH2=CH2��+H2O��
��3��װ��I��������SO2���Թ���Ʒ����Һ��ɫ��˵������SO2���ʴ�Ϊ������Ʒ����Һ��ɫ��
��4��װ�â�ͨ���������������Һ��ɫ������ϩ��������е�Ʒ�첻��ɫ�����е����Ը��������Һ��ɫ����֤������ϩ���ɣ�
�ʴ�Ϊ�����е�Ʒ�첻��ɫ�����е����Ը��������Һ��ɫ��
���� ���⿼������ϩ��ʵ�����Ʒ��Լ�����ļ��顢���������ȡ�����ʣ���Ŀ�Ѷ��еȣ�ע��������ϩ��ʵ������ȡԭ��������������ʣ���ȷ���ж��ֲ��������ʱ��Ӧ�����Ⱥ�˳����ȷ������ؼ�������������������������ϩ���ǽ����Ĺؼ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �����ƣ�aq�� | B�� | ����أ�aq�� | C�� | �����ƣ�s�� | D�� | ˮ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ʯ�ҡ����ס���ʯ�� | B�� | Һ̬����������Һ̬���� | ||
| C�� | �����ɱ����Ȼ������� | D�� | ��������ơ���ʽ̼��ͭ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �٢� | B�� | �٢� | C�� | �� | D�� | �٢ڢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 500 mL����ƿ | B�� | ��ͷ�ι� | C�� | �Թ� | D�� | ������ƽ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com