
 2012)����2016��1��1�����ҹ�ȫ��ʵʩ���ݴ�,������������ָ��(AQI)�ձ���ʵʱ���������SO2��NO2��CO��O3��PM10��PM2.5��ָ��,Ϊ�����ṩ����ָ��,�������ؾ���������ų��к����
2012)����2016��1��1�����ҹ�ȫ��ʵʩ���ݴ�,������������ָ��(AQI)�ձ���ʵʱ���������SO2��NO2��CO��O3��PM10��PM2.5��ָ��,Ϊ�����ṩ����ָ��,�������ؾ���������ų��к����| ������ | �ð�ˮ��SO2ת��(NH4)2SO3,��������(NH4)2SO4 |
| ������ | ���������Ƚ���(��Ҫ�ɷ�CO��CH4��H2)��SO2�ڸ����»�ԭ�ɵ����� |
| ������ | ��Na2SO3��Һ����SO2,�پ����ת��ΪH2SO4 |
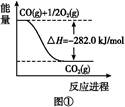
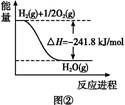

 2NO ��-746.5
2NO ��-746.5 N2(g)+2CO2(g)�Ħ�H="[-393.5��2-180.5-(-221.0)]kJ/mol=-746.5" kJ/mol��
N2(g)+2CO2(g)�Ħ�H="[-393.5��2-180.5-(-221.0)]kJ/mol=-746.5" kJ/mol��

 �ƸԹھ��ο���ϵ�д�
�ƸԹھ��ο���ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
 2NH3����Ӧ��1mol N2�ų�������Ϊ92.4kJ����Ͽ�1mol N��N�������յ������ǣ� ��
2NH3����Ӧ��1mol N2�ų�������Ϊ92.4kJ����Ͽ�1mol N��N�������յ������ǣ� ��| A��431kJ | B��945.6kJ | C��649kJ | D��869kJ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
 2CO(g)����H="-221.0" kJ��mol-1
2CO(g)����H="-221.0" kJ��mol-1 2H2O(g)����H="-483.6" kJ��mol-1
2H2O(g)����H="-483.6" kJ��mol-1 CO(g)+H2(g)�Ħ�HΪ(����)
CO(g)+H2(g)�Ħ�HΪ(����)| A��+131.3 kJ��mol-1 | B��-131.3 kJ��mol-1 | C��-352.3 kJ��mol-1 | D��+262.6 kJ��mol-1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
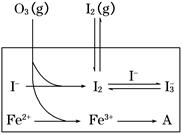
 HOI(aq)����H2
HOI(aq)����H2 I2(aq)��H2O(l)����H3
I2(aq)��H2O(l)����H3 I3��(aq)����ƽ�ⳣ������ʽΪ________��
I3��(aq)����ƽ�ⳣ������ʽΪ________��| ��� | ��Ӧ�� | ��ӦǰpH | ��Ӧ��pH |
| ��1�� | O3��I�� | 5.2 | 11.0 |
| ��2�� | O3��I����Fe2�� | 5.2 | 4.1 |
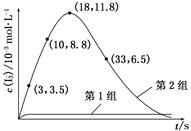
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
 2SO3(g)��H="-197" kJ��mol-1��ʵ����4 mol SO2�μ�������Ӧ�ų�354 kJ��������SO2��ת������ӽ���( )
2SO3(g)��H="-197" kJ��mol-1��ʵ����4 mol SO2�μ�������Ӧ�ų�354 kJ��������SO2��ת������ӽ���( )| A��90% | B��80% | C��50% | D��40% |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��������������Ӧ�����Ȼ���������Ȼ�ѧ����ʽ��H2��g����Cl2��g��=2HCl��g�� |
| B��������������Ӧ����2 mol�Ȼ������壬��Ӧ�Ħ�H����183 kJ��mol��1 |
| C��������������Ӧ����2 mol�Ȼ������壬��Ӧ�Ħ�H����183 kJ��mol��1 |
| D��������������Ӧ����1 mol�Ȼ������壬��Ӧ�Ħ�H����183 kJ��mol��1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
 O2(g)
O2(g) H2O(l)��H="-285.8" kJ/mol
H2O(l)��H="-285.8" kJ/mol CO2(g)+2H2O(l)��H="-890.3" kJ/mol
CO2(g)+2H2O(l)��H="-890.3" kJ/mol| A��1��1 | B��1��3 | C��1��4 | D��2��3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
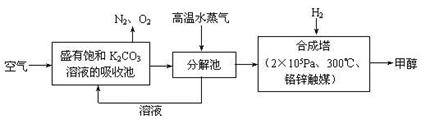
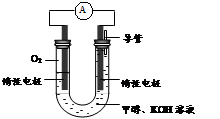
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com