
| A���٢ܢ� | B���ڢۢ� |
| C���ڢۢ� | D���ڢܢ� |


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��5.0 | B��7.0 |
| C��1.0 | D��14 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
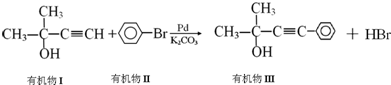

 �ķ�Ӧ������
�ķ�Ӧ�������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A���٢ڢ� | B���ڢۢ� |
| C���٢� | D���ڢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
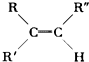
| [O] |
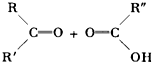 ���ж�ϩ��C10H18������KMnO4��Һ���ú�ɵõ������л����CH3��2CO��CH3COOH��CH3CO��CH2��2-COOH���ɴ��ƶϴ˶�ϩ���ܵĽṹ��ʽΪ��������
���ж�ϩ��C10H18������KMnO4��Һ���ú�ɵõ������л����CH3��2CO��CH3COOH��CH3CO��CH2��2-COOH���ɴ��ƶϴ˶�ϩ���ܵĽṹ��ʽΪ��������| A����CH3��2C�TC��CH3��CH2CH2CH�TCH CH2CH3 |
| B����CH3��2C�TCHCH2CH2C��CH3���TCHCH3 |
| C��CH3CH�TC��CH3��CH2CH2CH2C��CH3���TCH2 |
| D��CH3CH�TC��CH3��CH2CH2CH2CH�TCHCH3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ���� |
| ���� |
| ���� |
| ��ȡ |
| ��Һ |
| ���� |
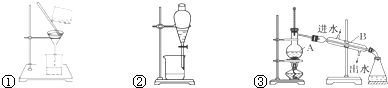
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A�������£�pH=7��NH4Cl��NH3?H2O�����Һ�У�c��NH4+��=c��Cl-�� |
| B������NaOH��Һ��ȥMgCl2��Һ��������FeCl3 |
| C������������ϡ���ᷴӦ��Fe+4H++NO3-=Fe3++2H2O+NO�� |
| D����ij��Һ�μ������ữ���Ȼ�����Һ�����ְ�ɫ��������֤������Һ�к���SO42- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A���ڢ� | B���٢� |
| C���٢ڢ� | D���٢ڢۢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��AlCl3��Һ�еμӹ�����ˮ��Al3++4OH-�TAlO2-+2H2O |
| B��������KMnO4��Һ��ͨ��SO2��3SO2+2MnO4-+4OH-=2MnO2��+3SO42-+2H2O |
| C������ʯ��ˮ�м�����С�մ���Һ��2HCO3-+Ca2++2OH-�TCaCO3��+2H2O+CO32- |
| D��������Һ��Cu��OH��2��Ӧ��Cu��OH��2+2H+�TCu2++2H2O |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com