
 Ϊ��̽�����������Ư�����õ����Ƕ������������Ƕ���������ˮ���õIJ��ijѧϰС���������ͼװ��������ʵ�飮��ش�������⣮
Ϊ��̽�����������Ư�����õ����Ƕ������������Ƕ���������ˮ���õIJ��ijѧϰС���������ͼװ��������ʵ�飮��ش�������⣮| c��SO32-�� | c��HSO3-�� | c��H2SO3�� | ��ɫ�ٶ� | |
| Na2SO3��Һ | �� | С | С | �� |
| NaHSO3��Һ | ������______ | ������______ | ������______ | �� |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
 ��2012?����һģ��ij�о���ѧϰС�����о���������Ư������ʱ���ӡ�������Ư������ʵ������������ˮ��Ӧ���ɵĴ������Ư�����á��õ�������Ϊ��̽�������������õõ����Ƕ������������Ƕ���������ˮ���õIJ����С�����������ʵ�飮��ش�������⣮
��2012?����һģ��ij�о���ѧϰС�����о���������Ư������ʱ���ӡ�������Ư������ʵ������������ˮ��Ӧ���ɵĴ������Ư�����á��õ�������Ϊ��̽�������������õõ����Ƕ������������Ƕ���������ˮ���õIJ����С�����������ʵ�飮��ش�������⣮�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ij�о���ѧϰС�����о���������Ư������ʱ���ӡ�������Ư������ʵ������������ˮ��Ӧ���ɵĴ������Ư�����á��õ�������Ϊ��̽�������������õõ����Ƕ������������Ƕ���������ˮ���õIJ����С�����������ʵ�飮��ش�������⣮
ij�о���ѧϰС�����о���������Ư������ʱ���ӡ�������Ư������ʵ������������ˮ��Ӧ���ɵĴ������Ư�����á��õ�������Ϊ��̽�������������õõ����Ƕ������������Ƕ���������ˮ���õIJ����С�����������ʵ�飮��ش�������⣮�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������һģ ���ͣ��ʴ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012���㽭ʡ�����и߿���ѧһģ�Ծ��������棩 ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012���㽭ʡ�����и�����һ�θ߿���ѧ������⻯ѧ�Ծ� ���ͣ�ʵ����
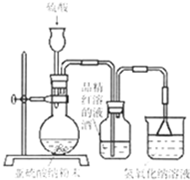
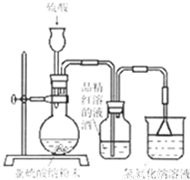
��8�֣�ij�о���ѧϰС�����о���������Ư������ʱ���ӡ�������Ư������ʵ������������ˮ��Ӧ���ɵĴ������Ư�����á��õ�������Ϊ��̽�������������õõ����Ƕ������������Ƕ���������ˮ���õIJ����С�����������ʵ�顣��ش�������⡣
��1��ʵ�������������Ʒ�ĩ��������ȡ��������������������������Һ��Ӧѡ�� ������ĸ���������� ��
A��98%Ũ���� B��70%���� C��10%ϡ����
��2��Ϊ��̽��SO2�ܷ�ʹƷ����ɫ����ͬѧѡ������ȷ��ҩƷ�����������ͼ��ʾʵ��װ�ã���ָ��ʵ��װ������еIJ�����֮����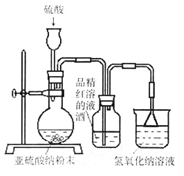
�� ���� ��
��3����ͬѧѡ������ȷװ�ú�ʵ���п��ƶ��������Դ�Լÿ��3�����ݵ��ٶ�ͨ��Ʒ��ľƾ���Һʱ������һСʱ��Ʒ���Բ���ɫ��Ϊ�ˣ�����ΪʹƷ���ˮ��Һ��ɫ���������� ��
��4����ͬѧ��һ��ʵ�����£�ȡ������ͬŨ�ȵ�Ʒ��ˮ��Һ����֧�Թ��У��ٷֱ���������������ƹ�������������ƹ��壬��֧�Թ��е�Ʒ�춼��ɫ�����ó��Ľ��ۣ�ʹƷ����ɫ�����϶���HSO3-��SO32-������Ϊ���Ľ����Ƿ���ȷ ���������� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com