
��֪��T��C��һ��ѹǿ�£����ݻ��ɱ���ܱ������г���2molSO2��1molO2����ʱ�����ݻ�ΪVL�����ֺ��º�ѹ��ʹ��Ӧ��2SO2��g��+O2��g��![]() 2SO3��g����5minĩ�ﵽƽ�⣬���SO3���������Ϊ0.4���Իش��������⣺��1��ƽ����������SO2���������Ϊ ��ƽ�������O2��ת����Ϊ ����2����ѡһ�ݻ��̶�������������Կ����¶�ΪT��C������2molSO2��1molO2ʹ��Ӧ���ﵽƽ��ʱSO3�����������Ϊ0.4�����ܱ����������Ϊ�� L��
2SO3��g����5minĩ�ﵽƽ�⣬���SO3���������Ϊ0.4���Իش��������⣺��1��ƽ����������SO2���������Ϊ ��ƽ�������O2��ת����Ϊ ����2����ѡһ�ݻ��̶�������������Կ����¶�ΪT��C������2molSO2��1molO2ʹ��Ӧ���ﵽƽ��ʱSO3�����������Ϊ0.4�����ܱ����������Ϊ�� L��
 �㾦�½̲�ȫ�ܽ��ϵ�д�
�㾦�½̲�ȫ�ܽ��ϵ�д� Сѧ�̲���ȫ���ϵ�д�
Сѧ�̲���ȫ���ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��6�֣���֪��T��C��һ��ѹǿ�£����ݻ��ɱ���ܱ������г���2molSO2��1molO2����ʱ�����ݻ�ΪVL�����ֺ��º�ѹ��ʹ��Ӧ��2SO2��g��+O2��g�� 2SO3��g����5minĩ�ﵽƽ�⣬���SO3���������Ϊ0.4���Իش��������⣺��1��ƽ����������SO2���������Ϊ ��ƽ�������O2��ת����Ϊ ����2����ѡһ�ݻ��̶�������������Կ����¶�ΪT��C������2molSO2��1molO2ʹ��Ӧ���ﵽƽ��ʱSO3�����������Ϊ0.4�����ܱ����������Ϊ�� L��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010���㽭ʡ���ݵ�����У�����߶��ڶ�ѧ�����л�ѧ�Ծ� ���ͣ�������
��6�֣���֪��T��C��һ��ѹǿ�£����ݻ��ɱ���ܱ������г���2molSO2��1molO2����ʱ�����ݻ�ΪVL�����ֺ��º�ѹ��ʹ��Ӧ��2SO2��g��+O2��g�� 2SO3��g����5minĩ�ﵽƽ�⣬���SO3���������Ϊ0.4���Իش��������⣺��1��ƽ����������SO2���������Ϊ ��ƽ�������O2��ת����Ϊ ����2����ѡһ�ݻ��̶�������������Կ����¶�ΪT��C������2molSO2��1molO2ʹ��Ӧ���ﵽƽ��ʱSO3�����������Ϊ0.4�����ܱ����������Ϊ�� L��
2SO3��g����5minĩ�ﵽƽ�⣬���SO3���������Ϊ0.4���Իش��������⣺��1��ƽ����������SO2���������Ϊ ��ƽ�������O2��ת����Ϊ ����2����ѡһ�ݻ��̶�������������Կ����¶�ΪT��C������2molSO2��1molO2ʹ��Ӧ���ﵽƽ��ʱSO3�����������Ϊ0.4�����ܱ����������Ϊ�� L��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013�����ʡ�ʹ�һ�и�����ѧ�ڵ�һ�μ�⿼�����ۻ�ѧ�Ծ����������� ���ͣ������
��14�֣�ˮú����һ�ָ�Ч����ȼ�ϣ�����Ҫ�ɷ���CO��H2������ˮ����ͨ�����ȵ�̿�Ƶã�C (s) + H2O(g) CO (g) +H2 (g) ��H��+131kJ?mol��1
CO (g) +H2 (g) ��H��+131kJ?mol��1
��T�¶��£��ĸ������о�������������Ӧ����������̿�������������ʵ����ʵ���Ũ�ȼ����淴Ӧ���ʹ�ϵ���±���ʾ������д������Ӧ�Ŀո�
| ���� ��� | c(H2O) /mol��L��1 | c(CO) /mol��L��1 | c(H2) /mol��L��1 | v����v���Ƚ� |
| I | 0.06 | 0.60 | 0.10 | v����v�� |
| II | 0.06 | 0.50 | 0.40 | �� |
| III | 0.12 | 0.40 | 0.80 | v����v�� |
| IV | 0.12 | 0.30 | �� | v����v�� |
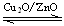 CH3OH(g) ��H��0���ϳɼ״���Ӧ��ϵ��ͨ������CO��ƽ���� �ƶ�����Сѹǿ��ƽ���� �ƶ��������¶���ƽ���� �ƶ��������ң�����
CH3OH(g) ��H��0���ϳɼ״���Ӧ��ϵ��ͨ������CO��ƽ���� �ƶ�����Сѹǿ��ƽ���� �ƶ��������¶���ƽ���� �ƶ��������ң������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010���㽭ʡ���ݵ�����У�����߶��ڶ�ѧ�����л�ѧ�Ծ� ���ͣ�������
��6�֣���֪��T��C��һ��ѹǿ�£����ݻ��ɱ���ܱ������г���2molSO2��1molO2����ʱ�����ݻ�ΪVL�����ֺ��º�ѹ��ʹ��Ӧ��2SO2��g��+O2��g�� 2SO3��g����5minĩ�ﵽƽ�⣬���SO3���������Ϊ0.4���Իش��������⣺��1��ƽ����������SO2���������Ϊ
��ƽ�������O2��ת����Ϊ
����2����ѡһ�ݻ��̶�������������Կ����¶�ΪT��C������2molSO2��1molO2ʹ��Ӧ���ﵽƽ��ʱSO3�����������Ϊ0.4�����ܱ����������Ϊ��
L��
2SO3��g����5minĩ�ﵽƽ�⣬���SO3���������Ϊ0.4���Իش��������⣺��1��ƽ����������SO2���������Ϊ
��ƽ�������O2��ת����Ϊ
����2����ѡһ�ݻ��̶�������������Կ����¶�ΪT��C������2molSO2��1molO2ʹ��Ӧ���ﵽƽ��ʱSO3�����������Ϊ0.4�����ܱ����������Ϊ��
L��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com