
����Ŀ��ij�¶��£���һ�ܱ������г���һ����CO2���������������ۣ�������Ӧ��Fe(s)��CO2(g) ![]() FeO(s)��CO(g)�����CO2��COŨ����ʱ��ı仯��ͼ��ʾ��
FeO(s)��CO(g)�����CO2��COŨ����ʱ��ı仯��ͼ��ʾ��
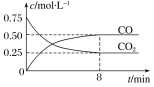
��1��0��8 min��v(CO2)��__________mol��L��1��min��1��
��2���÷�Ӧ�ں��º��ݵ��ܱ������н��У���˵���÷�Ӧ�ﵽ��ѧƽ��״̬����_____
A����λʱ���� ��ÿ����1molCO2ͬʱ����1molCO
B�������������ѹǿ������ʱ��仯
C��������������ܶȲ�����ʱ��仯
D�������������ƽ����Է�������������ʱ��仯
��3��������¶��£���ӦFe(s)��CO2(g) ![]() FeO(s)��CO(g)��ƽ�ⳣ��__________
FeO(s)��CO(g)��ƽ�ⳣ��__________
��4�����д�ʩ�У��ܹ��ı�ƽ��ʱc(CO)/c(CO2)�ı�ֵ����________(�����)��
A���¶� B�����۵���(����) C��ѹǿ D��CO����
��5����֪����ӦFe(s)��CO2(g) ![]() FeO(s)��CO(g)��ƽ�ⳣ��ΪK1����ӦFe(s)��H2O(g)
FeO(s)��CO(g)��ƽ�ⳣ��ΪK1����ӦFe(s)��H2O(g) ![]() FeO(s)��H2(g)��ƽ�ⳣ��ΪK2����ͬ�¶�ʱK1��K2��ֵ���±���
FeO(s)��H2(g)��ƽ�ⳣ��ΪK2����ͬ�¶�ʱK1��K2��ֵ���±���
�¶�/K | K1 | K2 |
973 | 1.47 | 2.38 |
1 173 | 2.15 | 1.67 |
�ٷ�ӦFe(s)��H2O(g) ![]() FeO(s)��H2(g)Ϊ______(����ȡ����ȡ�)��Ӧ
FeO(s)��H2(g)Ϊ______(����ȡ����ȡ�)��Ӧ
�ڸ��ݱ������ݣ����㷴ӦCO2(g)��H2(g) ![]() CO(g)��H2O(g) 973 K��K______��д������ʽ���ɣ����ؼ���������
CO(g)��H2O(g) 973 K��K______��д������ʽ���ɣ����ؼ���������
���𰸡�0.0625CD2A����1.47/2.38
��������
�⣺(1)0��8min��v(CO)=![]() =
=![]() =0.0625mol/(L��min)���ʴ�Ϊ��0.0625��
=0.0625mol/(L��min)���ʴ�Ϊ��0.0625��
(2)A����λʱ���� ��ÿ����1molCO2ͬʱ����1molCO������ʾ��������Ӧ���ʣ�����˵�����淴Ӧ�����Ƿ���ȣ���A����B����Ӧǰ����������ʵ������䣬�����������ѹǿʼ�ղ��䣬��B����C����ӦǰΪ������̼����Ӧ����һ����̼��������������ܶȲ�����ʱ��仯��˵��������̼��һ����̼�����ʵ���֮�Ȳ��䣬˵���ﵽ��ƽ��״̬����C��ȷ��D����Ӧǰ����������ʵ������䣬������������仯����������������ƽ����Է�������������ʱ��仯��˵���������䣬�ܹ�˵���ﵽƽ��״̬����D��ȷ����ѡCD��
(3)Fe(s)+CO2(g)FeO(s)+CO(g)��K1=![]() =
=![]() =2���ʴ�Ϊ��2��
=2���ʴ�Ϊ��2��
(4)A�������¶Ȼ��¶ȣ�ƽ��һ�������ƶ�����ñ�ֵһ�������仯����A��ȷ��B��Fe��Ϊ���壬���۵����ı䣬���ı�Ũ�ȣ�ƽ�ⲻ�ƶ�����ñ�ֵ�������仯����B����C����Ӧǰ����������ʵ������䣬�ı�ѹǿ��ƽ�ⲻ�ƶ�����ñ�ֵ�������仯����C����D���ı�CO����ƽ�ⷢ���ƶ������¶Ȳ��䣬K���䣬���Ըñ�ֵ���䣬��D��ȷ����ѡA��
(5)�����ݱ������ݣ���ӦFe(s)��H2O(g) ![]() FeO(s)��H2(g)��ƽ�ⳣ��ΪK2���¶����ߣ�ƽ�ⳣ����С��˵��ƽ�������ƶ�������ӦΪ���ȷ�Ӧ���ʴ�Ϊ�����ȣ�
FeO(s)��H2(g)��ƽ�ⳣ��ΪK2���¶����ߣ�ƽ�ⳣ����С��˵��ƽ�������ƶ�������ӦΪ���ȷ�Ӧ���ʴ�Ϊ�����ȣ�
��i��Fe(s)+CO2(g)FeO(s)+CO(g)��K1=![]() ��ii��Fe(s)+H2O(g)FeO(s)+H2(g)��K2=
��ii��Fe(s)+H2O(g)FeO(s)+H2(g)��K2=![]() ������ʽi-ii��CO2(g)+H2(g)CO(g)+H2O(g)��K=
������ʽi-ii��CO2(g)+H2(g)CO(g)+H2O(g)��K=![]() =
=![]() ���¶�Ϊ973Kʱ��K=
���¶�Ϊ973Kʱ��K=![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����2L�ܱ������ڣ�800��ʱ��Ӧ��2NO(g)+O2(g)![]() 2NO2(g)��ϵ�У�n(NO)��ʱ��ı仯�����
2NO2(g)��ϵ�У�n(NO)��ʱ��ı仯�����
ʱ��(s) | 0 | 1 | 2 | 3 | 4 | 5 |
n(NO)(mol) | 0.020 | 0.01. | 0.008 | 0.007 | 0.007 | 0.007 |
��1����֪��K300����K350����д���÷�Ӧ��ƽ�ⳣ������ʽ��K=_________________�����ڸ÷�Ӧ������˵���У���ȷ����________��
A����H>0����S>0 B����H>0����S<0
C����H<0����S<0 D����H<0����S>0
��2����ͼ�б�ʾNO2�ı仯��������____________________����O2��ʾ��0-2s�ڸ÷�Ӧ��ƽ������v=_______________��
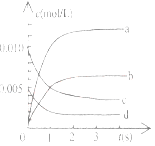
��3����˵���÷�Ӧ�Ѵﵽƽ��״̬����__________��
A��v(NO2)=2v(O2) B��������ѹǿ���ֲ���
C��v (NO)=2v��O2�� D���������ܶȱ��ֲ���
��4�����д�ʩ����ʹn(NO2)/n(NO)�������____��(����ĸ)
A�������¶� B���������
C�����ϳ���O2 D������He(g)��ʹ��ϵ��ѹǿ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������(Na2FeO4)��һ������Ч��ܾ�ˮ�������ŨNaOH��Һ�Ʊ��������Ƶ�װ����ͼ����ʾ������˵������ȷ����(����)
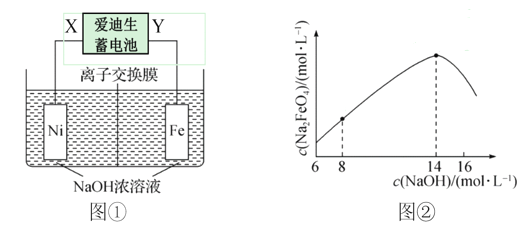
A. XΪ��Դ���������缫�����ĵ缫��Ӧʽ�ǣ�Fe - 6e- + 8OH- = FeO42- + 4H2O
B. ���ӽ���ĤΪ�����ӽ���Ĥ
C. ������1.66 gNa2FeO4ʱ���ռ����������ڱ�״���µ������672 mL
D. NaOH��Һ�ij�ʼŨ����������ɵ�Na2FeO4Ũ�ȵı仯��ϵ��ͼ����ʾ��c(Na2FeO4)���͵�ԭ������Ƿ����˸���Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������̬����ɵĻ��������ȫȼ�պ����õ�CO2��H2O�����ʵ��������������ʵ����ı仯��ͼ��ʾ�������йػ�������˵������ȷ����
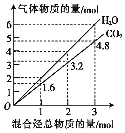
A. �û�������п��ܺ�����ϩ���Ȳ��C3H4��
B. �û��������һ�����м���
C. ��110�������£����������������ϣ��ܻ����ȼ��ǰ���������
D. �����������CH4��C2H4��ɣ����������Ϊ1��3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����й�������Ӫ�����ʵ�˵����ȷ����
A.������Է���ˮ�ⷴӦB.��֬���ڸ߷��ӻ�����
C.����Ӫ���ض������л���D.Cu2+�ж�������ʹ�����ʱ����й�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����к͵ζ����ⶨij�ռ���Ʒ�Ĵ��ȣ��Ը���ʵ��ش��������⣺
��1��ȷ����8.2 g�������������������ʵ���Ʒ�����500 mL������Һ������ʱ����Ʒ�ɷ���_________(������ĸ)������
A��С�ձ��� B���ྻֽƬ�ϡ� C��������
��2���ζ�ʱ����0.2000 mol/L�������Һ���ζ�������Һ����ѡ��_______(������ĸ)��ָʾ����
A������ B��ʯ�� C����̪
��3���ζ������У��۾�Ӧע��_______________________________�����÷�̪��ָʾ�����ζ��յ�ı�־��_____________________________________________��
��4�������±����ݣ����㱻���ռ���Һ�����ʵ���Ũ����________mol/L���ռ���Ʒ�Ĵ�����_____________��
�ζ� ���� | ������Һ ���(mL) | ������� | |
�ζ�ǰ�Ŀ̶�(mL) | �ζ���Ŀ̶�(mL) | ||
��һ�� | 10.00 | 0.40 | 20.50 |
�ڶ��� | 10.00 | 4.10 | 24.00 |
��5����δ�ô���Һ��ϴʢ�ű�����ĵζ��ܣ����ʹ�ⶨ���______����ƫ�ߡ���ƫ�͡�����Ӱ�족����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����1��������ʳ������Ӫ���DZ�֤���彡����������������Ҫ;����
����������Ԫ�����벻���Ӱ�����������������������������ܴﵽĿ�ĵ���______��
a����������ʳ�üӵ��� b����������ʹ����ǿ������ c��������������������
����������8�ְ��������ͨ��ʳ�����룬ijƷ�Ʊ��ɺ��е�������������ת��Ϊ���������______��
a����ά�� b�������� c����֬
��������ʷ������Ҫ�Ŀ�������______��
a����ù�� b��ά���� c�����ܲ���
��2������������Ȼ�ĺ�г��Ӫ�찲ȫ����̬�����ѳ�Ϊȫ����Ĺ�ʶ��
����ͼ������ֻ250mL����ƿ�зֱ����CO2�Ϳ������ð׳��������һ��ʱ�������aƿ���¶ȼƶ����Եͣ���ʢ��CO2����ƿΪ______���a����b������
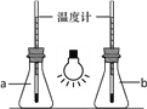
��Ϊ��ֹ��ɫ��Ⱦ���Ͼɵ�������Ͷ�뵽������ͼ��־������Ͱ�ڣ��ñ�־��ʾ����______��

�۷�ˮ����(Tl)���и߶��ԣ�����ʱ������NaClO��Һ����Tl+ת��ΪTl3+��NaClO��_____�������������ԭ��������ͬʱ����������ˮ����Tl3+ת��Ϊ������Tl(OH)3��д������Tl(OH)3�����ӷ���ʽ______��
��3��������������ᷢչ�����ʻ��������Ͽ�ѧ�ķ�չ�벻����ѧ��
��������մɵ�����ԭ�ϣ����н������˵ĸ������Ǻ������ʽ��ٵĸ�Ʒ�����������Ҫ��ɿɱ�ʾΪAl2Si2Ox(OH)4����x=______��
��2016����˻��ڰ������У����ݵĽ���������Ľ������ϡ���������ˮ�ࡢ������ͬ��ԭ����______�������ƣ��������ڵ�����ͨ���ø��ܶȾ���ϩ��HDPE��Ϊԭ���Ƶã�����ϩ����______���ϣ���ȹ��ԡ��������ԡ�������ϩ���ϳ�����ʳƷ��װ������ϩ�Ľṹ��ʽΪ_________����
�ۻ�ͨ��·�������ڻ����ƽ���������η�ֹ����ĸ�ʴ�ǹ��̼�����Ա���˵�����֮һ�����ڷ����绯ѧ��ʴʱ�ĸ�����Ӧ����ʽΪ______��
�ܹ�ҵ�ϳ���SiCl4��O2�ڸ���������ͨ���û���Ӧ�Ƶ�SiO2������2000�������ɹ���ϸ˿���÷�Ӧ�Ļ�ѧ����ʽΪ______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������£��� 0��01 mol��L��1NaOH��Һ�ζ� 20��00 mL 0��01 mol��L��1CH3COOH��Һ�����õζ�������ͼ������˵����ȷ����

A. a���Ӧ��Һ��pH=2
B. b���Ӧ����Һ�У�c(OH��)+ c(CH3COO��) = c(Na+)+ c(H+)
C. c���ʾNaOH��Һ��CH3COOH��Һǡ����ȫ��Ӧ
D. d���Ӧ����Һ�У�ˮ�ĵ���̶�С��ͬ���´�ˮ�ĵ���̶�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������Ȼ�ѧ����ʽ����ȷ���ǣ�������
A. 4g��������ȫȼ������SO2���ų�37 kJ������S(s)+O2(g)=SO2(g) ��H= -296kJ/mol
B. 1molN2��3molH2��ij�ܱ������з�Ӧ�ų�73kJ��������Ӧ���Ȼ�ѧ����ʽΪ��N2(g)+3H2(g)![]() 2NH3(g) ��H= -73kJ/mol
2NH3(g) ��H= -73kJ/mol
C. ����ı�ȼ����Ϊ-890.3kJ��mol-1�������ȼ�յ��Ȼ�ѧ����ʽ�ɱ�ʾΪ��CH4(g)+2O2(g) ==CO2(g)+ 2H2O(g) ��H=-890.3kJ��mol-1
D. ǿ��ǿ����к���Ϊ- 57.3 kJ/mol��Ba(OH) 2(aq)+H2SO4(aq)=BaSO4(S)+2H2O(l) ��H=-114.6kJ/mol
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com