
��ҵ���Ի�ͭ��(��Ҫ�ɷ�CuFeS2)Ϊԭ���Ʊ�����ͭ�����������ֹ��ա�
I.����������:���������Ļ�ͭ�����ʯӢ����ͨ�˿������б��գ������Ƶô�ͭ��
��1�����յ��ܷ�Ӧʽ�ɱ�ʾΪ��2CuFeS2 + 2SiO2+5O2��2Cu+2FeSi03+4SO2�÷�Ӧ����������_____��
��2�����д���SO2�ķ���������������_____
| A���߿��ŷ� |
| B���ô�����Һ�����Ʊ��������� |
| C���ð�ˮ���պ��پ������Ʊ������ |
| D����BaCl2��Һ�����Ʊ�BaSO3 |
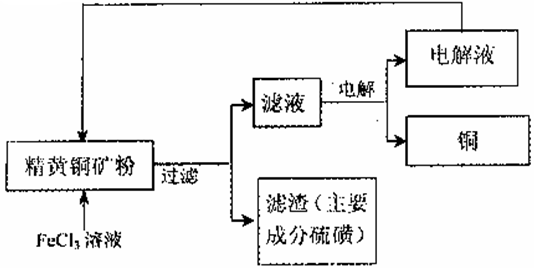
��15�֣���1��CuFeS2��O2����1�֣���2�֣�
��2��A��D�����1����1�֣���������֣���2�֣�
��3��C��2�֣�
��4��CuFeS2+4Fe3+��Cu2++5Fe2++S��û��ƽ��1�֣���3�֣�
��5��FeCl3����2�֣�
��6��Fe2+��e����Fe3+����2�֣�
��7��2.5��10��6��2�֣�
���������������1�����ݷ���ʽ2CuFeS2 + 2SiO2+5O2��2Cu+2FeSiO3+4SO2��֪���ڷ�Ӧ������Ԫ�صĻ��ϼ۴ӣ�2�����ߵ�+4�ۣ�ʧȥ���ӣ����CuFeS2�ǻ�ԭ������������Ԫ�صĻ��ϼ۴�0�۽��͵���2�ۣ�ͭԪ�صĻ��ϼ۴ӣ�2�۽��͵�0�ۣ���˸÷�Ӧ����������CuFeS2��O2��
��2��A.SO2�Ǵ�����Ⱦ����������ŷţ�A����ȷ��B.�ô�����Һ�����Ʊ��������ƣ����Է�ֹ��Ⱦ��B��ȷ��C.�ð�ˮ���պ��پ������Ʊ�����泥�Ҳ���Է�ֹ��Ⱦ��C��ȷ��D.SO2���������Ȼ�����Һ�У������BaCl2��Һ�����Ʊ�BaSO3�Dz����ܵģ�D����ȷ����ѡAD��
��3��¯����Ҫ�ɷ���FeO ��Fe2O3 ��SiO2 ,Al2O3�ȣ�Ϊ�õ�Fe2O3�������ܽ����Ҫ���˳��������衣Ȼ���������������Һ�е����������������������ӣ��ټ������������������Һ�����������������������ˡ�ϴ�����ռ��õ�����������˺�������������,δ�漰���IJ����������ᾧ����ѡC��
��4�����˵õ�����ֽ�к������ʣ���˵��CuFeS2��FeCl3��Һ��������������ԭ��Ӧ�������ӽ���������������˷�Ӧ�����ӷ���ʽΪCuFeS2+4Fe3+��Cu2++5Fe2++S��
��5�����ʱ�����ĵ��Һ�ֿ������ͭ��Ӧ����˸ù��������У�����ѭ�����õ�������FeCl3��
��6������������ʧȥ���ӣ��������ʯī�缫�����Һ������������������ʧȥ���ӣ����������ĵ缫��ʽFe2+��e����Fe3+��
��7����֪KSP(PbCl2)��1��10һ5����˵���Һ��c(C1һ)��2mo1��L-1ʱ��Һ��Pb2+���ʵ���Ũ��Ϊ ��2.5��10��6mol��L-1��
��2.5��10��6mol��L-1��
���㣺����������ԭ��Ӧ�жϡ���������β����������ѧʵ����������Լ��绯ѧԭ����Ӧ�������


 ����������ϵ�д�
����������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ����������Ԫ��A��B��C��D��E��Ԫ�����ڱ��е�λ������ͼ��ʾ������AΪ�ؿ��к�����ߵĽ���Ԫ�ء�
����������Ԫ��A��B��C��D��E��Ԫ�����ڱ��е�λ������ͼ��ʾ������AΪ�ؿ��к�����ߵĽ���Ԫ�ء�
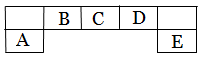
���û�ѧ����ش��������⣺ ��1��DԪ�������ڱ��е�λ�ã�
��1��DԪ�������ڱ��е�λ�ã�
��2��A��D ��EԪ�ؼ����Ӱ뾶�ɴ�С��˳��Ϊ_____��______ ��______ (�������� ) ��3��F��Dͬ���������ڣ�����̬�⻯���ȶ��ԵĴ�С �� ���������ţ�
��3��F��Dͬ���������ڣ�����̬�⻯���ȶ��ԵĴ�С �� ���������ţ�
��4���ø����������京��10���ӵ�DԪ���⻯�����ʱ��һ���������ͷ�һ�����ӣ�ͬʱ����һ�־��нϸ������Ե������ӣ���д���������ӵĵ���ʽ �����������д��ڵĻ�ѧ���� �� ��5��CԪ�صļ��⻯����EԪ�ص�����������ˮ���ﷴӦ�����ɻ�����K����K��ˮ��Һ��_____�ԣ�����ԡ��������ԡ������ԡ����������ӷ���ʽ��ʾ��ԭ�� ��
��5��CԪ�صļ��⻯����EԪ�ص�����������ˮ���ﷴӦ�����ɻ�����K����K��ˮ��Һ��_____�ԣ�����ԡ��������ԡ������ԡ����������ӷ���ʽ��ʾ��ԭ�� ��
��6�� ������AC�����Ժã�������ϵ��С�������õ����ȳ�����ϡ������Ʊ�AC��һ�ַ���Ϊ����AԪ�ص��������̿��C�ĵ�����1600 ~ 1750������AC��ÿ����1 mol AC������18 g̼������b kJ������������������Ϊ25�桢101��3 kPa�����£�д���÷�Ӧ���Ȼ�ѧ����ʽ ��
������AC�����Ժã�������ϵ��С�������õ����ȳ�����ϡ������Ʊ�AC��һ�ַ���Ϊ����AԪ�ص��������̿��C�ĵ�����1600 ~ 1750������AC��ÿ����1 mol AC������18 g̼������b kJ������������������Ϊ25�桢101��3 kPa�����£�д���÷�Ӧ���Ȼ�ѧ����ʽ ��
��7����Fe��Cu�Ļ�����м���һ����C������������ˮ����ϡ��Һ����ַ�Ӧ��ʣ�����m1 g���������м���ϡ���ᣬ��ַ�Ӧ����ʣ�� m2 g ������˵����ȷ���� ��
a������ϡ����ǰ�ͼ���ϡ��������Һ�п϶�����Cu2+
b������ϡ����ǰ�ͼ���ϡ��������Һ�п϶�����Fe2+
c��m1һ������m2
d��ʣ�����m1 g ��һ���е���ͭ��ʣ�����m2 g ��һ��û�е���ͭ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ҵ��������Ĺ�������ʾ��ͼ���£�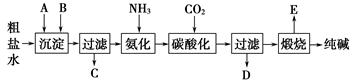
���������գ�
(1)����ˮ���������A��B������(������A��Դ��ʯ��Ҥ��)��д��A��B�Ļ�ѧʽ��
A______________��B____________��
(2)ʵ�����ᴿ���ε�ʵ���������Ϊ��
ȡ����__________��������__________��__________����ȴ�ᾧ��__________����ɡ�
(3)��ҵ��������������У�̼�ữʱ������������__________________��
̼�ữʱû������̼���ƾ��壬��ԭ����______________________��
(4)̼�ữ����ˣ���ҺD����Ҫ�ijɷ���______________(��д��ѧʽ)��������һ�ɷֵ������ӵľ��巽���ǣ�______________________��
(5)��������а���ѭ��ʹ�õģ�Ϊ�ˣ���ҺD����ʯ��ˮ����������ʯ��ˮ���������ķ�Ӧ�����ӷ���ʽΪ��____________________________��
(6)��Ʒ�����к���̼�����ơ�����ü��ȷֽ�ķ����ⶨ������̼�����Ƶ�����������������̼�����Ƶ����������ɱ�ʾΪ��__________(ע����ı���ʽ�����õ��йط��ŵĺ���)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ͭ����Ҫ�Ľ������ϡ�
(1)��ҵ�Ͽ���Cu2S��O2��Ӧ��ȡ��ͭ���÷�Ӧ��������Ϊ________������ͭ��ȡ��ͭ�����ʱ������������________�����Һ�б��뺬�е���������________��
(2)��100 mL 18 mol��L��1Ũ�����м��������ͭƬ������ʹ֮��ַ�Ӧ����Ӧ�б���ԭ��H2SO4Ϊ________mol��
(3)���ӹ�ҵ������������Ϊ30%��FeCl3��Һ��ʴ����ͭ���ľ�Ե����ӡˢ��·�壬Ϊ�˴�ʹ�ù��ķϸ�ʴҺ�л���ͭ�������µõ�FeCl3��Һ���������ʵ�����̡�
���������У������Լ��Ļ�ѧʽΪ��X________��Y________��Z________���ڢ���Ӧ�����ӷ���ʽΪ
___________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�������Ļ�����Ӧ�ù㷺����FeCl3������������ӡˢ��·ͭ�帯ʴ��������ֹѪ���ȡ�
��1��д��FeCl3��Һ��ʴӡˢ��·ͭ������ӷ���ʽ ��
| |
| | �������↑ʼ����ʱ��pH | �������������ȫʱ��pH |
| Fe3�� Fe2�� Cu2�� | 1.9 7.0 4.7 | 3.2 9.0 6.7 |
| �ṩ��ҩƷ��Cl2 ŨH2SO4 NaOH��Һ CuO Cu | ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
I ����ʵ�顱�ǽ�һ֧��С�IJ�������װ������һ�����������У�����װ�����ԭ����Ҫ�����������е�ʵ�顣������������ŵ㣬���㷺Ӧ���ڻ�ѧʵ���У���ͼʵ��Ϊ����ʵ�顱��С�Թ�������մ����ˮ����ͭ��ĩ��������۲�ʵ��װ�ã�����ʵ��ԭ�����ش��������⣺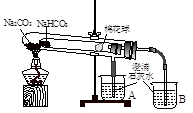
��1����ʵ���Ŀ����_____________________
��2��ʵ�鿪ʼǰ���Թܣ�˵��װ�ò�©����������
��3��һ��ʱ������ʵ�飬��װ����ȴ��ȡ��С�Թ��й�������ˮ��Ȼ��μ�1mol/L���ᣬ����CO2��������������Ĺ�ϵ��ͼ��ʾ�����к�������________________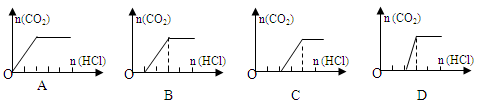
IIȼ�ϵ����һ�������Ľ�ȼ�Ϻ��������Ļ�ѧ��ֱ��ת��Ϊ���ܵĻ�ѧ��ء������������¡��״���Һ������壬��������ȼ�ϵ�ص�ȼ�ϡ���ش��������⣺
��1���Լ��������Ϊԭ�ϣ�����������ҺΪ�������Һ���ɵ�ء�д����������Ӧʽ
��2�����������Ϊ��Դ��ʯīΪ�缫���1L0. 1mol/L���Ȼ�����Һ���ش��������⣺
д������ܷ�Ӧ�����ӷ���ʽ 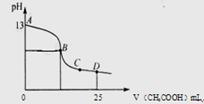
��3������ʱ�����һ��ʱ���ȡ25mL����������Һ���μ�0.2mol/L���ᣬ���������������Һ��pH�Ĺ�ϵ��ͼ��ʾ��������������ʧ����������ˮ����Һ����仯���Բ��ƣ�
�ټ������ı�״���¼��� mL
����ͼ�е�B��pH=7�������ǡ����ȫ��Ӧ�ĵ��� ���䣨�AB������BC����CD����
��AB������Һ�и�����Ũ�ȴ�С��ϵ�п�����ȷ����
A�� c(K+)��c(OH��)��c (CH3COO��) ��c(H+)
B�� c(K+)��c(CH3COO��)��c(OH��) ��c(H+)
C�� c(K+)��c(CH3COO��)=c(OH��) ��c(H+)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������(CH3OCH3)����ɫ���壬����Ϊһ��������Դ���ɺϳ���(���ΪH2��CO��������CO2)ֱ���Ʊ������ѣ����е���Ҫ���̰��������ĸ���Ӧ��
�״��ϳɷ�Ӧ��
(��)CO(g)��2H2(g)=CH3OH(g) ��H1����90.1 kJ��mol��1
(��)CO2(g)��3H2(g)=CH3OH(g)��H2O(g) ��H2����49.0 kJ��mol��1
ˮú���任��Ӧ��
(��)CO(g)��H2O(g)=CO2(g)��H2(g) ��H3����41.1 kJ��mol��1
�����Ѻϳɷ�Ӧ��
(��)2CH3OH(g)=CH3OCH3(g)��H2O(g) ��H4����24.5 kJ��mol��1
�ش��������⣺
(1)Al2O3�Ǻϳ���ֱ���Ʊ������ѷ�Ӧ��������Ҫ�ɷ�֮һ����ҵ�ϴ��������Ʊ��ϸߴ���Al2O3����Ҫ����������____________________________________________(�Ի�ѧ����ʽ��ʾ)��
(2)���������Ѻϳɷ�Ӧ(��)����COת���ʵ�Ӱ��
________________________________________________________________________��
(3)���о����ڴ���(��CuZnAlO��Al2O3)��ѹǿΪ5.0 MPa�������£���H2��COֱ���Ʊ������ѣ������ͼ��ʾ������COת�������¶����߶����͵�ԭ����_________________________________________________��
(4)������ֱ��ȼ�ϵ�ؾ��������졢Ч�ʸߵ��ŵ㣬�������ܶȸ��ڼ״�ֱ��ȼ�ϵ��(5.93 kW��h��kg��1)���������Ϊ���ԣ�������ֱ��ȼ�ϵ�صĸ�����ӦΪ__________
_____________________��һ�������ѷ��Ӿ����绯ѧ���������Բ���________________�����ӵĵ������õ�ص����������ѹΪ1.20 V�������ܶ�E��_______________(��ʽ���㡣�����ܶȣ�����������/ȼ��������1 kW��h��3.6��106 J)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ij��ѧ��ȤС�鰴���������̽��С���þ���Ͻ��Ʊ����������塱��ʵ�顣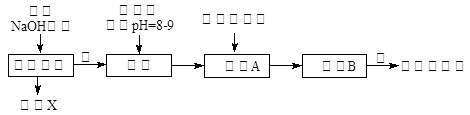
��1��þ���Ͻ��м�NaOH��Һ�����ӷ�Ӧ����ʽΪ ����������X��ԭ�ӽṹʾ��ͼ ������A�Ļ�ѧʽΪ ��
��2��д����������ˮ�еĵ��뷽��ʽ �������ڰ����IJ���������Ũ���� �����ˡ����
��3������ȤС��Ϊ�ⶨþ���Ͻ��и���ɵ����������������ͼװ�ã�����Ҫ�ⶨ�������� ���� �� 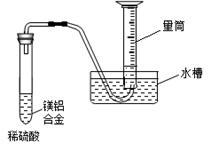
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com