
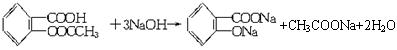
 (��Է�������Ϊ180)����֪����˾ƥ��ҩƬ��һ�����������ĸ���(��������ˮ��Ҳ����NaOH��Ӧ)��ij����ѧϰС��Ϊ���鰢˾ƥ����Ч�ɷֵĹ����Ų��ⶨ����ҩƬ�еĺ���������������ʵ�飺
(��Է�������Ϊ180)����֪����˾ƥ��ҩƬ��һ�����������ĸ���(��������ˮ��Ҳ����NaOH��Ӧ)��ij����ѧϰС��Ϊ���鰢˾ƥ����Ч�ɷֵĹ����Ų��ⶨ����ҩƬ�еĺ���������������ʵ�飺 ��.��֤����ˮ�����о����Ȼ������Ľṹ(��COOR)��
�ٽ�һƬ��˾ƥ�����������ˮ�����ã���ȡ��Һ2 mL����֧�ྻ���Թ��У�
����һ֧�Թ��м���____________�����۲쵽____________����֤������ˮ���������Ȼ���
������һ֧�Թ��м�������NaOH��Һ�����ȼ����ӣ���ȴ����ε���ϡH2SO4���������е���FeCl3��Һ���ӱ������۲쵽��Һ��Ϊ____________ɫ����֤������ˮ�����о������Ľṹ��
�ش��������⣺
(1)�����������հײ���������
(2)ʵ���еμ�ϡH2SO4��������_____________________________________________��
��.��ѧϰС�����������ʵ�鷽���ⶨ����ˮ������ҩƬ�еĺ�����
�ٳ�ȡ��˾ƥ����Ʒm g���ڽ���Ʒ���飬����V1 mL a mol��L-1 NaOH��Һ��(����)�����ȣ���ȥ���ϵȲ������������Һ������ƿ��������ƿ�еμӼ���ָʾ������Ũ��Ϊb mol��L-1���������ζ�δ��Ӧ��NaOH��������������ΪV2 mL��
�ش��������⣺
(1)��˾ƥ���м������NaOH��Һ�����ȷ�����Ӧ�Ļ�ѧ����ʽΪ____________�����м���Ŀ����____________________________________________________________��
(2)����������÷�̪��ָʾ��������жϵζ��յ㣿________________________________
____________________________________________________________________��
(3)����ʵ���м�¼�����ݣ���˾ƥ��ҩƬ������ˮ�������������ı���ʽΪ___________
_____________________________________________________________________��
��.(1)ʯ����Һ ��Һ��Ϊ��ɫ ��
(2)�к�NaOH��������ҺΪ������
��.(1)
ʹ����ˮ������ٷ�Ӧ
(2)��ƿ����Һ��ɫ��ȥ�Ұ�����ڲ��ٱ�Ϊ��ɫ
(3)��av1-bv2��10-3��60/m�ݡ�100%
��������.�Ȼ������ԣ�����ʯ����Һ���飻����ˮ������ɷ��ǻ�����FeCl3��Һ����ɫ����.����ˮ������NaOH����1��3�ı�����Ӧ������ˮ�����������������ʽΪ
![]() ��180��100%=
��180��100%=![]() ��100%��
��100%��


 ��У���˳�̾�ϵ�д�
��У���˳�̾�ϵ�д� ��У���һ��ͨϵ�д�
��У���һ��ͨϵ�д� �γ̴����Ծ�����100��ϵ�д�
�γ̴����Ծ�����100��ϵ�д� �¾�����ĩ���100��ϵ�д�
�¾�����ĩ���100��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
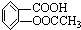 ����Է�������Ϊ180����
����Է�������Ϊ180����

| ����� | 0.1mol/L�������/mL | |
| �ζ�ǰ | �ζ��� | |
| 1 | 0.00 | 17.98 |
| 2 | 1.56 | 16.58 |
| 3 | 0.22 | 15.20 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
��֪���ٰ�˾ƥ��ҩƬ��һ�����������ĸ���(��������ˮ��Ҳ����NaOH��Ӧ)���ڷ���������FeCl3��Һ���������������ܷ�����ɫ��Ӧ��
ij����ѧϰС��Ϊ���鰢˾ƥ����Ч�ɷ��еĹ����Ų��ⶨ����ˮ������ҩƬ�еĺ���������������ʵ�飺
����֤����ˮ�����о����Ȼ������Ľṹ(��COOR)��
�ٽ�һƬ��˾ƥ�����������ˮ�����ã���ȡ��Һ2mL����֧�ྻ���Թ��У�����һ֧�Թ��м���___________�����۲쵽___________����֤������ˮ���������Ȼ���������һ֧�Թ��м�������NaOH��Һ�����ȼ����ӣ���ȴ����ε���ϡH2SO4���������е���FeCl3��Һ���ӱ������۲쵽��Һ��Ϊ___________ɫ����֤������ˮ�����о������Ľṹ��
�ش��������⣺
(1)�����������հײ���������
(2)ʵ���еμ�ϡH2SO4��������_________________________________��
���ѧϰС�����������ʵ�鷽���ⶨ����ˮ������ҩƬ�еĺ�����
�ٳ�ȡ��˾ƥ����Ʒmg���ڽ���Ʒ���飬����V1mLamol��L-1NaOH��Һ(����)�����ȣ���ȥ���ϵȲ������������Һ������ƿ��������ƿ�еμӼ���ָʾ������Ũ��Ϊbmol��L-1�ı����ᷴ��δ��Ӧ��NaOH��������������ΪV2mL��
�ش��������⣺
(1)��˾ƥ���м������NaOH��Һ�����ȷ�����Ӧ�Ļ�ѧ����ʽΪ___________________�����У����ȵ�Ŀ����______________________��
(2)���ܽ���˾ƥ���ܽ���ˮ��ֱ����NaOH��Һ���еζ���ԭ����__________________��
(3)����ʵ���м�¼�����ݣ���˾ƥ��ҩƬ������ˮ�������������ı���ʽΪ��____________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
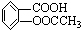
| ����� | 0.1mol/L�������/mL | |
| �ζ�ǰ | �ζ��� | |
| 1 | 0.00 | 17.98 |
| 2 | 1.56 | 16.58 |
| 3 | 0.22 | 15.20 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
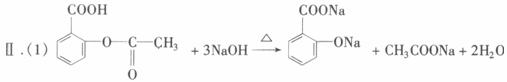
��֪���ٰ�˾ƥ��ҩƬ��һ�����������ĸ���(��������ˮ��Ҳ����NaOH��Ӧ)���ڷ���������FeCl3��Һ���������������ܷ�����ɫ��Ӧ��
ij����ѧϰС��Ϊ���鰢˾ƥ����Ч�ɷ��еĹ����Ų��ⶨ����ˮ������ҩƬ�еĺ���������������ʵ�飺
��.��֤����ˮ�����о����Ȼ������Ľṹ(��COOR)��
�ٽ�һƬ��˾ƥ�����������ˮ�����ã���ȡ��Һ2 mL����֧�ྻ���Թ��У�
����һ֧�Թ��м���_________�����۲쵽__________________����֤������ˮ���������Ȼ���
������һ֧�Թ��м�������NaOH��Һ�����ȼ����ӣ���ȴ�������ϡH2SO4���������е���FeCl3��Һ���ӱ������۲쵽��Һ��Ϊ_________ɫ����֤������ˮ�����о������Ľṹ��
�ش��������⣺
(1)�����������հײ���������
(2)ʵ���еμ�ϡH2SO4��������__________________��
��.��ѧϰС�����������ʵ�鷽���ⶨ����ˮ������ҩƬ�еĺ�����
�ٳ�ȡ��˾ƥ����Ʒm g��
�ڽ���Ʒ���飬����V1 mL a mol��L-1 NaOH��Һ(����)�����ȣ���ȥ���ϵȲ������������Һ������ƿ��
������ƿ�еμӼ���ָʾ������Ũ��Ϊb mol��L-1�ı����᷵��δ��Ӧ��NaOH��������������ΪV2 mL��
�ش��������⣺
(1)��˾ƥ���м������NaOH��Һ������ʱ������Ӧ�Ļ�ѧ����ʽΪ__________________;���У����ȵ�Ŀ����__________________________________________��
(2)���ܽ���˾ƥ���ܽ���ˮ��ֱ����NaOH��Һ���еζ���ԭ����_____________________
____________________________________________________________________��
(3)����ʵ���м�¼�����ݣ���˾ƥ��ҩƬ������ˮ�������������ı���ʽΪ_____________
_____________________________________________________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com