
����Ŀ��A��B��C��D���ַ����廯������ǵĽṹ��ʽ������ʾ��

����д���пհף�
(1)A�ķ���ʽΪ______________�� D�к��������ŵ�������____________��
(2)�������ֻ������У���Ϊͬ���칹�����______������ĸ���ţ���
(3)�����ڼ���A��C����______������ţ���
�����Ը��������Һ ��̼��������Һ ���Ȼ�����Һ
(4)DΪԭ�Ϻϳ�ij�������ϵ��������£�

��ʾ��R-CH2-COOH+Cl2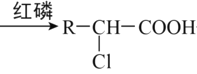 +HCl
+HCl
��D��E�ķ�Ӧ����_________________��
��F�Ľṹ��ʽ_________________��
��E��F��Ӧ�Ļ�ѧ����ʽ��________________��
��G��H��Ӧ�Ļ�ѧ����ʽ��_________________��
���𰸡�C11H14O2 �Ȼ� CD �� ȡ�� ![]()
![]() +2NaOH
+2NaOH![]()
![]() +NaCl+2H2O
+NaCl+2H2O ![]() +CH3OH
+CH3OH![]()
 +H2O
+H2O
��������
(1)������ṹ��ʽȷ�������ʽ��D�к��й�����Ϊ�Ȼ���
(2)����ʽ��ͬ�ṹ��ͬ�����ʻ�Ϊͬ���칹�壻
(3)A�к����ѻ���C�к��з��ǻ������ݹ����ŵ���ͬ�������
(4)�����������������Ϣ�϶�ȡ����Ӧ����E��E�Ľṹ��ʽΪ![]() ��E������ȥ��Ӧ����F��F�Ľṹ��ʽΪ
��E������ȥ��Ӧ����F��F�Ľṹ��ʽΪ![]() ��F���ữ����G��G�Ľṹ��ʽΪ
��F���ữ����G��G�Ľṹ��ʽΪ![]() ��G�ͼ״�����������Ӧ����H��H�Ľṹ��ʽΪ
��G�ͼ״�����������Ӧ����H��H�Ľṹ��ʽΪ ��
��
(1)������ṹ��ʽ֪�����ʽΪC11H14O2��������ṹ��ʽ֪D�к��������������Ȼ���
(2)����ʽ��ͬ�ṹ��ͬ�����ʻ�Ϊͬ���칹�壬���ݽṹ��ʽ֪��C��D��Ϊͬ���칹�壻
(3)A�к����ѻ���̼̼˫����C�к��з��ǻ���̼̼˫����
�ٶ��߶�����̼̼˫�������Զ���ʹ���Ը��������Һ��ɫ�����������𣬢ٴ���
�ڶ��߶�����̼��������Һ��Ӧ�����������𣬢ڴ���
��A�к����ѻ���C�к��з��ǻ������ܺ��Ȼ�����Һ������ɫ��Ӧ���ѻ������Ȼ�����Һ������ɫ��Ӧ�����������Ȼ�����Һ���𣬢���ȷ��
�ʺ���ѡ���Ǣۣ�
(4)�����������������Ϣ�϶�ȡ����Ӧ����E��E�Ľṹ��ʽΪ![]() ��E������ȥ��Ӧ����F��F�Ľṹ��ʽΪ
��E������ȥ��Ӧ����F��F�Ľṹ��ʽΪ![]() ��F���ữ����G��G�Ľṹ��ʽΪ
��F���ữ����G��G�Ľṹ��ʽΪ![]() ��G�ͼ״�����������Ӧ����H��H�Ľṹ��ʽΪ
��G�ͼ״�����������Ӧ����H��H�Ľṹ��ʽΪ ��
��
��ͨ�����Ϸ���֪�����������������ȡ����Ӧ����E��![]() ���÷�Ӧ����ȡ����Ӧ��
���÷�Ӧ����ȡ����Ӧ��
��ͨ�����Ϸ���֪��F�Ľṹ��ʽΪ![]() ��
��
��E��![]() ��������NaOH���Ҵ���Һ���ȣ�������ȥ��Ӧ����F��E��F��Ӧ�Ļ�ѧ����ʽ��
��������NaOH���Ҵ���Һ���ȣ�������ȥ��Ӧ����F��E��F��Ӧ�Ļ�ѧ����ʽ��![]() +2NaOH
+2NaOH![]()
![]() +NaCl+2H2O��
+NaCl+2H2O��
��G�Ľṹ��ʽΪ![]() ��G�ͼ״�CH3OH��Ũ������ڲ�����ʱ����������Ӧ����H���÷�Ӧ����ʽΪ��
��G�ͼ״�CH3OH��Ũ������ڲ�����ʱ����������Ӧ����H���÷�Ӧ����ʽΪ��![]() +CH3OH
+CH3OH![]()
 +H2O��
+H2O��


 ���Ͱ�ͨ�������Сѧ��ʱͬ�����ϵ�д�
���Ͱ�ͨ�������Сѧ��ʱͬ�����ϵ�д� ���Ͱ�ͨ������ϵ�д�
���Ͱ�ͨ������ϵ�д� �ٷ�ѧ����ҵ��������ϵ�д�
�ٷ�ѧ����ҵ��������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����л�����������ȷ����
A. 2���һ�����
2���һ�����
B.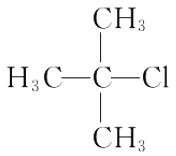 2������2���ȱ���
2������2���ȱ���
C.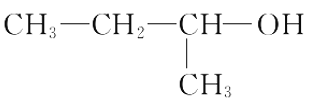 2������1������
2������1������
D. 2��2��3��������3����ϩ
2��2��3��������3����ϩ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������˵����ȷ����(����)
A. ![]() �����е�����ԭ���п��ܹ�ƽ��
�����е�����ԭ���п��ܹ�ƽ��
B. ![]() ����8��̼ԭ�ӿ�����ͬһ��ֱ����
����8��̼ԭ�ӿ�����ͬһ��ֱ����
C.  ����̼ԭ��һ����ͬһƽ����
����̼ԭ��һ����ͬһƽ����
D. ![]() ������16��ԭ�ӹ�ƽ��
������16��ԭ�ӹ�ƽ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��25��ʱ����25 mL 0.100 0 mol L-l��BOH��Һ����ε���ͬŨ�ȵ�һԪ����HA����Һ��������ҺPH�����HA��Һ������Ĺ�ϵ������ͼ��ʾ��������˵����ȷ���ǣ� ��
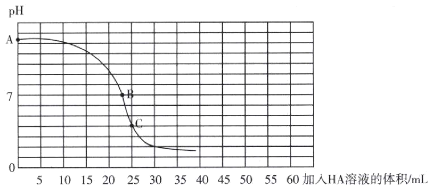
A.BOH�ĵ��뷽��ʽΪBOH =B++OH-
B.��ѡ���̪��ָʾ�����ζ��յ�ʱ����Һǡ����B�����ɫ��Ϊ�ۺ�ɫ����30���ڲ��ָ�ԭɫ
C.Ka(HA)��Kb(BOH)
D.����HA��Һ�����Ϊ50 mlʱ��c(B+)+2c(BOH)+c(OH-)=c(HA)+c(H+)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������������Ӽ�����1��2-�������顣��ͼΪʵ�����Ʊ�1��2-���������װ�Dͼ�� ͼ�з�Һ©������ƿa�зֱ�װ��ŨH2SO4����ˮ�Ҵ���dװ�D�Թ���װ��Һ�塣

��֪��CH3CH2OH![]() CH2=CH2��+H2O��
CH2=CH2��+H2O��
2CH3CH2OH![]() CH3CH2OCH2CH3+H2O
CH3CH2OCH2CH3+H2O
��������б����£�
�Ҵ� | 1��2-�������� | ���� | �� | |
״̬ | ��ɫҺ�� | ��ɫҺ�� | ��ɫҺ�� | ����ɫҺ�� |
�ܶ�/g��cm-3 | 0.79 | 2.18 | 0.71 | 3.10 |
�е�/�� | 78.5 | 131.4 | 34.6 | 58.8 |
�۵�/�� | -114.3 | 9.79 | - 116.2 | -7.2 |
ˮ���� | ���� | ���� | �� | ���� |
(1)��ȫƿb��ʵ�����ж������á���һ���Լ��ʵ�������dװ�D�е����Ƿ�����������д����������ʱƿb�е�����_______________�����ʵ��ʱdװ�D�е��ܶ���������Ϊ���ܵ�ԭ���Ǣ�_______________����ȫƿb�������������Ǣ�_______________��
(2)����c��e�ж�ʢ��NaOH��Һ��c��NaOH��Һ��������_____________________��
(3)ijѧ��������ʵ��ʱ��ʹ��һ������Һ�壬����ȫ����ɫʱ���������Ҵ���Ũ������Һ����������������³������࣬���װ�D��������û�����⣬�Է������ܵ�ԭ��______________��______________(д����������)��
(4)��ȥ����������δ��Ӧ��Br2�����е���Ҫ����Ϊ___________��Ҫ��һ���ᴿ�����в����б������_____________ (����ĸ)��
A���ؽᾧ B������ C����ȡ D������
(5)ʵ����Ҳ���Գ�ȥdװ�D��ʢ��ˮ���ձ�����Ϊ����ˮֱ�Ӽ��뵽dװ�D���Թ��У����ʱ��ˮ����������ȴ1��2-��������������⣬��������������______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������̼�IJ�����ת���ǿ�ѧ�о��е��ȵ����⡣�ҹ�������Ա�������Ni/Al2O3Ϊ�������� CO2(g)��H2(g)ת��ΪCH4(g)��H2O(g)�ķ�Ӧ���̣���ʾ��ͼ���£�

(1)�ÿ��淴Ӧ�Ļ�ѧ����ʽΪ_________��ʹ�ô���Ni/Al2O3_________ (������������������)���{CO2��ƽ��ת���ʡ�
(2)300���£���һ�����ܱ������г���һ������CO2��H2������������Ӧ��һ��ʱ���Ӧ��ƽ�⣬�������������䣬�¶ȴ�300������500�棬��Ӧ���´ﵽƽ��ʱ��H2������������ӡ�����˵���������_________(����)��
A.�÷�Ӧ��![]()
B.ƽ�ⳣ����С��![]()
C.300���£���С![]() ��ֵ��
��ֵ��![]() ��ƽ��ת��������
��ƽ��ת��������
D.��Ӧ�ﵽƽ��ʱ��![]()
(3)��һ�������£���Ӧ��ϵ��CO2��ƽ��ת����a( CO2)��L��X�Ĺ�ϵ��ͼ��ʾ��L��X��ʾ�¶Ȼ�ѹǿ��

��X��ʾ����������___________��
��L1_____________L2(����<����>��)���������______________________________________��
(4)��1 L�����ܱ������м���4.0 mol H2(g)��1.0 mol CO2����������(����Ϊ Ni/AI2O3���¶�ΪT1)ʹ֮����������Ӧ����������������ѹǿ��ʱ��ı仯��ͼ��ʾ��

��4 minʱCO2��ת����Ϊ___________��
��T1�¶��¸÷�Ӧ�Ļ�ѧƽ�ⳣ��Ϊ___________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��[��ѧ����ѡ��2����ѧ�뼼��]�ۺ���������PFS����ˮ��������Ҫ������������ͼ���Ի��շ���мΪԭ���Ʊ�PFS��һ�ֹ������̡�

�ش���������
��1������м��ҪΪ���渽�д�������������������Ҫ�ɷ�Ϊ_________�������ɸ��Ŀ����_______��
��2�����ʱ����ʵ�����_____��д���������ᷴӦ�����ӷ���ʽ_____________________��
��3����Ӧ���м�����������������_________������������������ʵ���____________�����ţ���
a��KMnO4b��![]() c��
c��![]() d��
d��![]()
��4���ۺϸ�����Һ��pH���������һ���ķ�Χ�ڡ�pHƫСʱFe3+ˮ��̶�����pHƫ��ʱ��_______��
��5������ڳ�ѹ��������ѹ�������ŵ���______��
��6���λ���B�Ǻ�������������Ч������Ҫָ�꣬����ʽΪ![]() ��nΪ���ʵ�������Ϊ������Ʒ��Bֵ��ȡ��Ʒmg��ȷ����������ᣬ��ַ�Ӧ���ټ�����к���ȴ������ˮ���Է�̪Ϊָʾ������c
��nΪ���ʵ�������Ϊ������Ʒ��Bֵ��ȡ��Ʒmg��ȷ����������ᣬ��ַ�Ӧ���ټ�����к���ȴ������ˮ���Է�̪Ϊָʾ������c![]() �ı�NaOH��Һ�����к͵ζ������ֲ�����ȥ�����ų������Ӹ��ţ������յ�ʱ����NaOH��ҺV mL�����������������հ������飬����NaOH��Һ
�ı�NaOH��Һ�����к͵ζ������ֲ�����ȥ�����ų������Ӹ��ţ������յ�ʱ����NaOH��ҺV mL�����������������հ������飬����NaOH��Һ![]() ����֪����Ʒ��Fe����������w����B�ı���ʽΪ__________��
����֪����Ʒ��Fe����������w����B�ı���ʽΪ__________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����.��ͼ��ʾ��ʵ���г��õļ��������������Ҫ����ա�
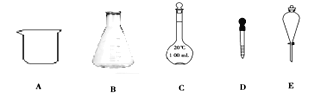
(1)���������������У�����һ�����ʵ���Ũ�ȵ���Һʵ������Ҫʹ����Щ������________������ţ�������Ҫʹ�õ������ǣ�_______�������ƣ���
(2)���й�������C��ʹ�÷����У���ȷ����________(������ѡ��ı��)��
a.ʹ��ǰӦ����Ƿ�©Һ b.ʹ��ǰ������
c.�����������ʷ�Ӧ���ܽ������ d.����Һ��ֱ��ת�Ƶ�����
��.ʵ����Ҫ����90 mL 0.100 mol��L��1 Na2CO3��Һ����ش��������⣺
(1)���ƹ�������Ҫ�õ���������������ƽ(�����룬��ȷ��0.1g)����ͷ�ιܡ���Ͳ��ҩ�ס��ձ��⣬����Ҫ�������У�________________��
(2)����Na2CO3��10H2O��������Һ����Ҫ�������������Ϊ________g��
(3)����������֪��̼���ƹ�����NaOH���ƣ��ڿ�����Ҫ���⣬�ڳ���ʱ����Ҫע��ѡ����___________ʢװNa2CO3���塣
(4)�����ƹ����У�����������������ƫ�ߵ���___________��
a.����ʧȥ�˲��ֽᾧˮ
b.����ʱ���ӿ̶���
c.δ��ȴ�����¾ͽ���Һת�Ƶ�����ƿ������
d.���ݺ�����ƿ����תҡ�ȣ����ú�Һ����ڿ̶��ߣ��ټ�ˮ���̶���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��2.0mol PC13��1.0mol Cl2�������������ܱ������У���һ�������·���������Ӧ: PCl3(g)+Cl2(g) ![]() PCl5(g)��ƽ��ʱ��PCl5Ϊ0.40mol�������ʱ����1.0mol PCl3��0.5mol Cl2������ͬ�¶����ٴ�ƽ��ʱPCl3�����ʵ�����
PCl5(g)��ƽ��ʱ��PCl5Ϊ0.40mol�������ʱ����1.0mol PCl3��0.5mol Cl2������ͬ�¶����ٴ�ƽ��ʱPCl3�����ʵ�����
A.0.40molB.0.20mol
C.����0.80mol����1.6molD.��0.80mol
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com