
ؤ³رذ¾؟ذشر§د°ذ،×éہûسأدآءذشءد؛حء÷³جضئ±¸CuSO4،¤5H2O،£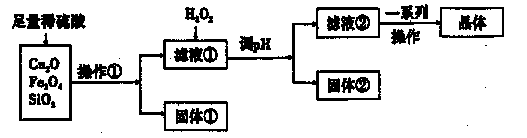
زرضھ£؛Cu+شعثلذشجُ¼دآ²»خب¶¨£¬ز×ةْ³ة½ًتôCu؛حCu2+،£
£¨1£©²ظ×÷¢ظµؤأû³ئخھ____،£ £¬
£¨2£©بô¹ججه¢ظµؤ³ة·ضخھ»ى؛دخشٍئن³ة·ضµؤ»¯ر§ت½خھ____،£
£¨3£©½«H2O2¼سبëآثز؛¢ظضذ£¬·¢ةْ·´س¦µؤہë×س·½³جت½خھ ،£
£¨4£©µ÷pH؟ةر،سأµؤز©ئ·خھ____،£
| A£®NaOHبـز؛ | B£®CuO | C£®°±ث® | D£®CuCO3 |
 أûذ£؟خجأدµءذ´ً°¸
أûذ£؟خجأدµءذ´ً°¸
| ؤ꼶 | ¸كضذ؟خ³ج | ؤ꼶 | ³ُضذ؟خ³ج |
| ¸كز» | ¸كز»أâ·ر؟خ³جحئ¼ِ£، | ³ُز» | ³ُز»أâ·ر؟خ³جحئ¼ِ£، |
| ¸ك¶ | ¸ك¶أâ·ر؟خ³جحئ¼ِ£، | ³ُ¶ | ³ُ¶أâ·ر؟خ³جحئ¼ِ£، |
| ¸كب | ¸كبأâ·ر؟خ³جحئ¼ِ£، | ³ُب | ³ُبأâ·ر؟خ³جحئ¼ِ£، |
؟ئؤ؟£؛¸كضذ»¯ر§ ہ´ش´£؛ جâذح£؛جî؟صجâ
FeCl3شعدض´ْ¹¤زµةْ²ْضذس¦سأ¹م·؛،£ؤ³»¯ر§رذ¾؟ذشر§د°ذ،×éؤ£ؤ⹤زµةْ²ْء÷³جضئ±¸خقث®FeCl3£¬شظسأ¸±²ْئ·FeCl3بـز؛خüتصسذ¶¾µؤH2S،£
¢ٌ.¾²éشؤ×تءدµأضھ£؛خقث®FeCl3شع؟صئّضذز׳±½â£¬¼سببز×ة»ھ،£ثûأاةè¼ئءثضئ±¸خقث®FeCl3µؤتµرé·½°¸£¬×°ضأت¾زâح¼£¨¼سبب¼°¼ذ³ض×°ضأآشب¥£©¼°²ظ×÷²½ضèبçدآ£؛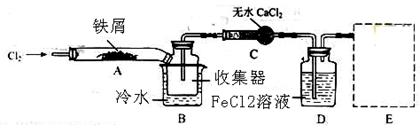
¢ظ¼ى²é×°ضأµؤئّأـذش£»
¢عح¨بë¸ةشïµؤCl2£¬¸د¾،×°ضأضذµؤ؟صئّ£»
¢غسأ¾ئ¾«µئشعجْذ¼دآ·½¼سببضء·´س¦حê³ة
¢ـ£®£®£®£®£®£®£®£®
¢فجهدµہنب´؛َ£¬ح£ض¹ح¨بëCl2£¬²¢سأ¸ةشïµؤN2¸د¾،Cl2£¬½«تص¼¯ئ÷أـ·â
اë»ط´ًدآءذختجâ£؛
£¨1£©×°ضأAضذ·´س¦µؤ»¯ر§·½³جت½خھ ،£
£¨2£©µع¢غ²½¼سبب؛َ£¬ةْ³ةµؤرج×´FeCl3´َ²؟·ض½ّبëتص¼¯ئ÷£¬ةظء؟³ء»شع·´س¦¹ـAسز¶ثزھت¹³ء»µؤFeCl3½ّبëتص¼¯ئ÷£¬µع¢ـ²½²ظ×÷تا ،£
£¨3£©²ظ×÷²½ضèضذ£¬خھ·ہض¹FeCl3³±½âثù²ةب،µؤ´ëت©سذ£¨جî²½ضèذٍ؛إ£© ،£
£¨4£©×°ضأDضذFeCl2ب«²؟·´س¦؛َ£¬زٍت§ب¥خüتصCl2µؤ×÷سأ¶ّت§ذ§£¬ذ´³ِ¼ىرéFeCl2تا·ٌت§ذ§µؤتش¼ء£؛ ،£
£¨5£©شعذéدك؟ٍضذ»³ِخ²ئّخüتص×°ضأE²¢×¢أ÷تش¼ء،£
¢ٍ£®¸أ×éح¬ر§سأ×°ضأDضذµؤ¸±²ْئ·FeCl3بـز؛خüتصH2S£¬µأµ½µ¥ضتءٍ£»¹آث؛َ£¬شظزشت¯ؤ«خھµç¼«£¬شعز»¶¨جُ¼دآµç½âآثز؛،£
£¨6£©FeCl3سëH2S·´س¦µؤہë×س·½³جت½خھ ،£
£¨7£©µç½â³طضذH+شعزُ¼«·إµç²ْةْH2£¬رô¼«µؤµç¼«·´س¦ت½خھ ،£
£¨8£©×غ؛د·ضخِتµرé¢ٍµؤء½¸ِ·´س¦£¬؟ةضھ¸أتµرéسذء½¸ِدشضّسإµم£؛
¢ظH2Sµؤش×سہûسأآتخھ100%£»¢ع ،£
²é؟´´ً°¸؛ح½âخِ>>
؟ئؤ؟£؛¸كضذ»¯ر§ ہ´ش´£؛ جâذح£؛جî؟صجâ
دض´ْةç»لضذحشع²»ح¬ءىسٍسذ¹م·؛µؤس¦سأ،£ؤ³ح؟َت¯؛¬رُ»¯ح،¢رُ»¯راح،¢برُ»¯¶جْ؛حآِت¯£¨SiO2)£¬دض²ةسأثل½·¨´س؟َت¯ضذجلب،ح£¬ئن¹¤زصء÷³جح¼بçدآ،£ئنضذحµؤفحب،£¨ح´سث®²م½ّبëسذ»ْ²مµؤ¹³ج£©؛ح·´فحب،£¨ح´سسذ»ْ²م½ّبëث®²مµؤ¹³ج£©تادض´ْتھ·¨ء¶حµؤضطزھ¹¤زصتض¶خ،£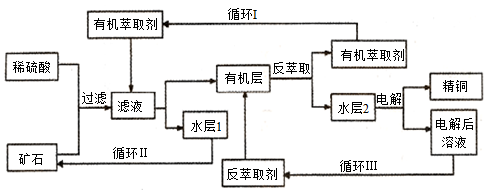
زرضھ£؛·´فحب،؛َµؤث®²م2تاءٍثلحبـز؛،£»ط´ًدآءذختجâ£؛
(1£©؟َت¯سأد،ءٍثل´¦ہي¹³جضذ·¢ةْ¶à²½·´س¦£¬³ءث·¢ةْCu2O+2H+£½Cu2++Cu+H2O؛حFe2O3+6H+£½2Fe3++3H2O·´س¦ح⣬ئنثü·´س¦µؤہë×س·½³جت½خھ____________________________________________،£
(2)¼ىرéآثز؛ضذتا·ٌ؛¬سذFe3+µؤ·½·¨تا____________________________________________________،£
(3)،°ر»·I،±¾¶à´خر»·؛َµؤث®²م1²»ؤـ¼جذّر»·ت¹سأ£¬µ«؟ة·ضہë³ِز»ضضضطزھµؤءٍثلرخ£¬بôث®²م1±©آ¶شع؟صئّضذز»¶خت±¼ن؛َ£¬؟ةزشµأµ½ءيز»ضضضطزھµؤءٍثلرخ£¬ذ´³ِث®²مl±©آ¶شع؟صئّضذ·¢ةْ·´س¦µؤہë×س·½³جت½________ _____________،£
(4£©ذ´³ِµç½â¹³جضذرô¼«£¨¶èذشµç¼«£©·¢ةْ·´س¦µؤµç¼«·´س¦ت½ __________________،£
(5)،°ر»·¢َ،±ضذ·´فحب،¼ءµؤض÷زھ³ة·ضµؤ»¯ر§ت½تا________________،£
(6) »ئح؟َ£¨ض÷زھ³ة·ضCuFeS2£©تاجلب،حµؤض÷زھشءد£¬؟ة²ةسأ»ً·¨بـء¶¹¤زصةْ²ْح£¬¸أ¹¤زصµؤضذ¼ن¹³ج»ل·¢ةْ·´س¦£؛Cu2S£«2Cu2O£½6Cu+SO2،ü،£¸أ·´س¦ضذ£¬_______ (جر§ت½)×÷»¹ش¼ء£¬أ؟ةْ³ة1mol Cu£¬·´س¦ضذ×ھزئµç×سµؤخïضتµؤء؟خھ____________،£
²é؟´´ً°¸؛ح½âخِ>>
؟ئؤ؟£؛¸كضذ»¯ر§ ہ´ش´£؛ جâذح£؛جî؟صجâ
آءتاز»ضضس¦سأ¹م·؛µؤ½ًتô£¬¹¤زµةدسأAl2O3؛ح±ù¾§ت¯£¨Na3AlF6£©»ى؛دبغبعµç½âضئµأ،£
¢ظآءحء؟َµؤض÷زھ³ة·ضتاAl2O3؛حSiO2µب،£´سآءحء؟َضذجلء¶Al2O3µؤء÷³جبçدآ£؛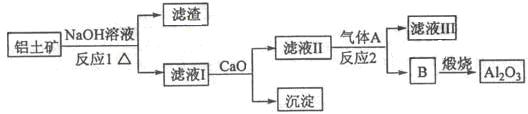
¢عزشس©ت¯£¨CaF2£©؛ح´؟¼îخھشءدضئ±¸±ù¾§ت¯µؤء÷³جبçدآ£؛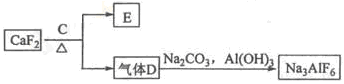
»ط´ًدآءذختجâ£؛
£¨1£©ذ´³ِ·´س¦1µؤ»¯ر§·½³جت½ £»
£¨2£©آثز؛¢ٌضذ¼سبëCaOةْ³ةµؤ³ءµيتا £¬·´س¦2µؤہë×س·½³جت½خھ £»
£¨3£©E؟ة×÷خھ½¨ض²ؤءد£¬»¯؛دخïCتا £¬ذ´³ِسةDضئ±¸±ù¾§ت¯µؤ»¯ر§·½³جت½ £»
£¨4£©µç½âضئآءµؤ»¯ر§·½³جت½تا £¬زشت¯ؤ«خھµç¼«£¬رô¼«²ْةْµؤ»ى؛دئّجهµؤ³ة·ضتا ،£
²é؟´´ً°¸؛ح½âخِ>>
؟ئؤ؟£؛¸كضذ»¯ر§ ہ´ش´£؛ جâذح£؛جî؟صجâ
´سآءحء؟َ£¨ض÷زھ³ة·ضتا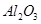 £¬؛¬
£¬؛¬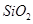 ،¢
،¢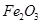 ،¢MgOµبشسضت£©ضذجلب،ء½ضض¹¤زصئ·µؤء÷³جبçدآ£؛
،¢MgOµبشسضت£©ضذجلب،ء½ضض¹¤زصئ·µؤء÷³جبçدآ£؛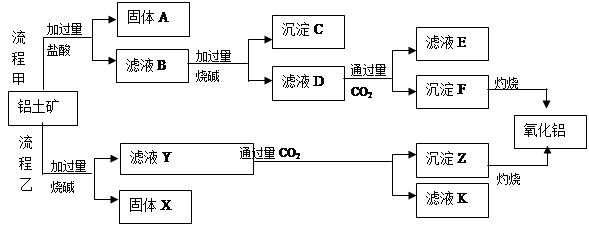
اë»ط´ًدآءذختجâ£؛
£¨1£©ء÷³جزز¼سبëةص¼î؛َµؤہë×س·½³جت½خھ_________________________________________.
£¨2£©¹ججهAµؤس¦سأ_________________________________________.£¨ء½µم£©
£¨3£©آثز؛Dسëةظء؟CO2·´س¦µؤہë×س·½³جت½خھ__________________________________£¬
دٍ¸أآثز؛Kضذ¼سبë×مء؟ت¯»زث®µؤہë×س·½³جت½تا________
£¨4£©ء÷³جززضئرُ»¯آءµؤسإµمتاثùسأµؤتش¼ء½د¾¼أ£¬ب±µمتا__________________________
£¨5£©زرضھ298Kت±£¬ µؤبـ¶ب»³£ت
µؤبـ¶ب»³£ت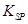 =10-11,ب،تتء؟µؤآثز؛B,¼سبëز»¶¨ء؟µؤةص¼îا،ت¹أ¾ہë×س³ءµيحêب«£¬شٍبـز؛µؤPH×îذ،خھ_______.
=10-11,ب،تتء؟µؤآثز؛B,¼سبëز»¶¨ء؟µؤةص¼îا،ت¹أ¾ہë×س³ءµيحêب«£¬شٍبـز؛µؤPH×îذ،خھ_______.
²é؟´´ً°¸؛ح½âخِ>>
؟ئؤ؟£؛¸كضذ»¯ر§ ہ´ش´£؛ جâذح£؛جî؟صجâ
¹¤زµضئ±¸آب»¯حت±£¬½«إ¨رخثلسأصôئّ¼سببضء80 ،و×َسز£¬آآ¼سبë´ضCuO·غؤ©(؛¬شسضتFe2O3،¢FeO)£¬³ن·ض½ء°è£¬ت¹ض®بـ½â£¬µأز»ا؟ثلذشµؤ»ى؛دبـز؛£¬دضسû´س¸أ»ى؛دبـز؛ضذضئ±¸´؟¾»µؤCuCl2بـز؛£¬²ةسأزشدآ²½ضè[²خ؟¼ت¾ف£؛pH،ف9.6ت±£¬Fe2£«حêب«ث®½â³ةFe(OH)2£»pH،ف6.4ت±£¬Cu2£«حêب«ث®½â³ةCu(OH)2£»pH،ف3.7ت±£¬Fe3£«حêب«ث®½â³ةFe(OH)3]،£اë»ط´ًزشدآختجâ£؛
£¨1£©µعز»²½³ب¥Fe2£«£¬ؤـ·ٌض±½سµ÷صûبـز؛pH£½9.6£¬½«Fe2£«³ءµي³ب¥£؟ £¬£¨جî،°ؤـ،± »ٍ،°²»ؤـ،±£©ہيسةتا £¬
£¨2£©سذبثسأا؟رُ»¯¼ءNaClOدب½«Fe2£«رُ»¯خھFe3£«£¬شظµ÷صûبـز؛µؤPH½«Fe3£«³ءµي³ب¥£؛
¢ظ¼سبëNaClO؛َ£¬بـز؛µؤpH±ن»¯تا________(جî´ْ؛إ)،£
A£®ز»¶¨شِ´َ B£®ز»¶¨¼ُذ، C£®؟ةؤـشِ´َ D£®؟ةؤـ¼ُذ،
¢عؤمبدخھسأNaClO×÷رُ»¯¼ءتا·ٌح×µ±£؟ £¬£¨جî،°تا،± »ٍ،°·ٌ،±£©دضسذدآءذ¼¸ضض³£سأµؤرُ»¯¼ء£¬؟ةسأسع³ب¥»ى؛دبـز؛ضذFe2£«µؤسذ________(سذ¼¸¸ِر،¼¸¸ِ£¬جî´ْ؛إ)،£
A£®إ¨HNO3 B£®Cl2 C£®KMnO4 D£®H2O2
£¨3£©¼سبëتتµ±خïضتµ÷صûبـز؛pH£¬ت¹Fe3+×ھ»¯خھFe(OH)3³ءµي£¬؟ةزش´ïµ½³ب¥Fe3+¶ّ²»ثًت§CuCl2µؤؤ؟µؤ£¬شٍµ÷صûبـز؛pH؟ةر،سأدآءذخïضتضذµؤ___________،£
A£®NaOH B£®NH3،¤H2O C£®CuO D£®Cu(OH)2
²é؟´´ً°¸؛ح½âخِ>>
؟ئؤ؟£؛¸كضذ»¯ر§ ہ´ش´£؛ جâذح£؛جî؟صجâ
آب»¯آءبعرخµç½â·¨تازشآب»¯آءخھشءد£¬زش¼î½ًتô»ٍ¼îحء½ًتôآب»¯خ؛¬ةظء؟MgCl2،¢KCl،¢CaCl2£©خھµç½âضت½ّذذµç½âضئب،آءµؤ·½·¨،£
£¨1£©آب»¯آءبعرخµç½â·¨ذèزھدبضئ´؟رُ»¯آء،£زشآءحء؟َ£¨ض÷زھ³ة·ضخھAl2O3£¬؛¬سذةظء؟Fe2O3،¢SiO2µبشسضت£©خھشءدح¨¹زشدآح¾¾¶جل´؟رُ»¯آء£؛
¢ظزہ´خذ´³ِX،¢Yµؤ³ة·ض £¬ ،£
¢عبçح¼ثùت¾شعتµرéتزضذ½ّذذ¹آث£¬²ظ×÷ضذµؤء½´¦´يخَ·ض±ًتا £» ،£
£¨2£©ضئ±¸خقث®آب»¯آءµؤ·´س¦خھ£؛2Al2O3+6Cl2 4AlCl3+3O2
4AlCl3+3O2
¢غخھ´ظ½ّ¸أ·´س¦µؤ½ّذذ£¬تµ¼تةْ²ْضذذè¼سبë½¹ج؟£¬ئنشہيتا ،£
¢ـ¼سبë½¹ج؟؛َµؤ»¯ر§·´س¦؟ة±يت¾خھAl2O3+C+Cl2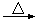 AlCl3+X،ü£¬خھب·¶¨ئّجهXتا·ٌتا»ى؛دئّجه£¬ؤ³ح¬ر§½«Xزہ´خح¨¹×ئببµؤرُ»¯ح؛ح³خاهµؤت¯»زث®£¬شظ¸ù¾فدضدَإذ¶د،£¸أ²ظ×÷تا·ٌصب·£؟£¨جîصب·،¢²»صب·»ٍخق·¨إذ¶د£© £¬اëثµأ÷ہيسة ،£
AlCl3+X،ü£¬خھب·¶¨ئّجهXتا·ٌتا»ى؛دئّجه£¬ؤ³ح¬ر§½«Xزہ´خح¨¹×ئببµؤرُ»¯ح؛ح³خاهµؤت¯»زث®£¬شظ¸ù¾فدضدَإذ¶د،£¸أ²ظ×÷تا·ٌصب·£؟£¨جîصب·،¢²»صب·»ٍخق·¨إذ¶د£© £¬اëثµأ÷ہيسة ،£
£¨3£©دضشع¹¤زµةدح¨³£سأµç½âبغبعرُ»¯آء·½ت½ضئب،آء£¬ہيآغةدةْ²ْ1¶ضآءدû؛ؤµؤرُ»¯آءµؤضتء؟_________
£¨جî،°´َسع،±،¢،°ذ،سع،±،¢،°µبسع،±£©آب»¯آءضتء؟،£
²é؟´´ً°¸؛ح½âخِ>>
؟ئؤ؟£؛¸كضذ»¯ر§ ہ´ش´£؛ جâذح£؛جî؟صجâ
°رةظء؟½ًتôؤئح¶بëدآءذبـز؛ضذ£¬ذ´³ِسذ¹ط·´س¦µؤ»¯ر§·½³جت½£؛
£¨1£©ؤئح¶بëد،رخثلضذ ،£
£¨2£©ؤئح¶بëءٍثلحبـز؛ضذ ،£
²é؟´´ً°¸؛ح½âخِ>>
؟ئؤ؟£؛¸كضذ»¯ر§ ہ´ش´£؛ جâذح£؛¼ئثمجâ
زش¸»؛¬ءٍثلراجْµؤ¹¤زµ·دز؛خھشءدةْ²ْرُ»¯جْµؤ¹¤زصبçدآ(²؟·ض²ظ×÷؛حجُ¼آش)£؛
¢ٌ.´س·دز؛ضذجل´؟²¢½ل¾§³ِFeSO4،¤7H2O،£
¢ٍ.½«FeSO4،¤7H2Oإنضئ³ةبـز؛،£
¢َ.FeSO4بـز؛سëةش¹ء؟µؤNH4HCO3بـز؛»ى؛د£¬µأµ½؛¬FeCO3µؤ×از؛،£
¢ô.½«×از؛¹آث£¬سأ90 ،وببث®د´µس³ءµي£¬¸ةشï؛َµأµ½FeCO3¹ججه،£
¢ُ.ىرةصFeCO3£¬µأµ½Fe2O3¹ججه،£
زرضھ£؛NH4HCO3شعببث®ضذ»ل·ض½â،£
¢إ¢ٌضذ£¬¼س×مء؟µؤجْذ¼³ب¥·دز؛ضذµؤFe3£«£¬¸أ·´س¦µؤہë×س·½³جت½تا ،£
¢ئ¢ٍضذ£¬ذè¼سز»¶¨ء؟ثل£¬¸أثل×î؛أتا ،£شثسأ»¯ر§ئ½؛âشہيزش¼°ہë×س·½³جت½¼ٍتِ¸أثلµؤ×÷سأ ،£
¢ا¢َضذ£¬ةْ³ةFeCO3µؤہë×س·½³جت½تا ،£بôFeCO3×از؛³¤ت±¼ن±©آ¶شع؟صئّضذ£¬»لسذ²؟·ض¹ججه±يأو±نخھ؛ى؛ضة«£¬¸أ±ن»¯µؤ»¯ر§·½³جت½تا ،£
(4)¢ôضذ£¬ح¨¹¼ىرéSO42-ہ´إذ¶د³ءµيتا·ٌد´µس¸ة¾»£¬¼ىرéSO42-µؤ²ظ×÷تا
(5)زرضھىرةصFeCO3µؤ»¯ر§·½³جت½تا4FeCO3£«O2 =2Fe2O3£«4CO2،£دضىرةص464.0 kgµؤFeCO3£¬µأµ½316.8 kg²ْئ·،£بô²ْئ·ضذشسضتض»سذFeO£¬شٍ¸أ²ْئ·ضذFe2O3µؤضتء؟تا kg،£
²é؟´´ً°¸؛ح½âخِ>>
°ظ¶بضآذإ - ء·د°²لءذ±ي - تشجâءذ±ي
؛±±ت،»¥ءھحّخ¥·¨؛ح²»ء¼ذإد¢¾ظ±¨ئ½ج¨ | حّةدسذ؛¦ذإد¢¾ظ±¨×¨اّ | µçذإص©ئ¾ظ±¨×¨اّ | ةوہْت·ذéخقض÷زهسذ؛¦ذإد¢¾ظ±¨×¨اّ | ةوئَاضب¨¾ظ±¨×¨اّ
خ¥·¨؛ح²»ء¼ذإد¢¾ظ±¨µç»°£؛027-86699610 ¾ظ±¨ستدن£؛58377363@163.com