
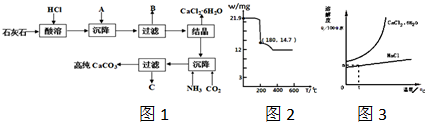
 ��
��| 21.9g |
| 219g/mol |
| 14.7g-11.1g |
| 18g/mol |
 ��CaCl2��
��CaCl2�� ��CaCl2
��CaCl2


 ��һ������ĩ�ٷֳ�̾�ϵ�д�
��һ������ĩ�ٷֳ�̾�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
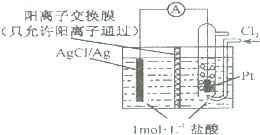
| A��������ӦΪCl2-2e-=2Cl- |
| B���ŵ�ʱH+���Ҳྭ�����ӽ���Ĥ������� |
| C����·��ת��0.01 mole-ʱ������Ĥ�����Һ�м���0.01 mol���� |
| D������NaCl��Һ�������ᣬ��ص��ܷ�Ӧ����ı� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| �������� | ��ʼ���ݵ�pH | ������ȫ��pH |
| Fe3+ | 1.1 | 3.2 |
| Fe2+ | 5.8 | 9.6 |
| Co2+ | 5.8 | 9.4 |
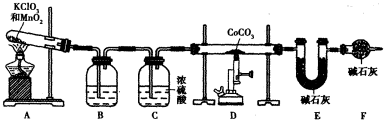
| x |
| y |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
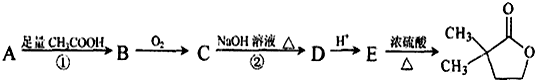
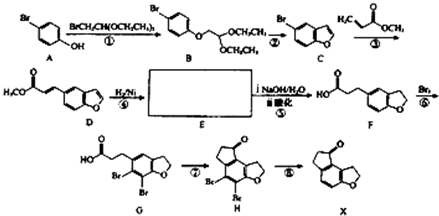
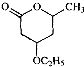 ��B��һ����Ҫ��ͬ���칹�壮����ƺ�����������ɴ�
��B��һ����Ҫ��ͬ���칹�壮����ƺ�����������ɴ�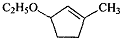 ��
��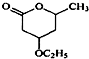 �ĺϳ�·�ߣ�������ͼ��ʾ����ע����Ӧ��������
�ĺϳ�·�ߣ�������ͼ��ʾ����ע����Ӧ��������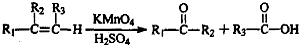 ��
��| Ũ���� |
| 170�� |
| ���¡���ѹ |
| ���� |
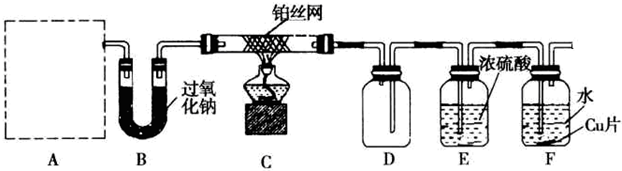
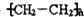 ��
��
| H2O |
| ���� |
�鿴�𰸺ͽ���>>
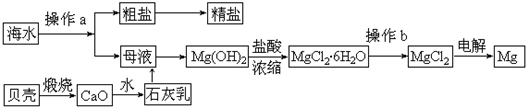
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ���¸�ѹ |
| ���� |
 B��
B��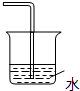 C��
C�� D��
D��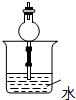
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A�����͡������Ҵ��������������� |
| B����5��̼ԭ�ӵ��л�������������γ�5��̼̼���� |
| C�������ʵ������Ҵ���������ȫȼ��ʱ����������������� |
| D��������ϩ����ʹ��ˮ��ɫ�����߷�Ӧԭ����ͬ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com