
| A��δϴ���ܽ�NaCl���ձ� |
| B��������ƿ����Һʱ��������Һ�彦�� |
| C������ʱ���ӿ̶��� |
| D������ƿ������ϴ�Ӻ������������ˮ |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��Ħ���ǹ��ʵ�λ���аѺ�����ʵ������������ӵ���Ŀ��ϵ������һ�������� |
| B��Ħ�������ʵ����ĵ�λ�����Ħ������Ϊmol |
| C��Ħ���������Ǹ����ʵ���Է������������ԭ������ |
| D�������ӵ�����Ϊ6.02��1023 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��1mol Cu������ϡ���ᷴӦ����NA��NO���� |
| B����״���£�2.24 L CHCl3�ķ�����Ϊ0.1 NA |
| C��t��ʱ��1L pH=6�Ĵ�ˮ�У���1��10-6NA��OH- |
| D����FeI2��Һ��ͨ����������������1mol Fe2+������ʱ���ܹ�ת�Ƶ��ӵ���ĿΪ1 NA |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����״���£�22.4LH2O����ԭ�Ӹ���Ϊ3NA |
| B��1.7g��������ԭ����ĿΪ0.4NA |
| C��ͬ��ͬѹʱ����ͬ������κ����嵥��������ԭ������ͬ |
| D��1 L 1mol��L-1��Na2SO4��Һ�к�Na+�ĸ���ΪNA |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��ԭ���� | B�����ʵ��� | C���ܶ� | D�������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
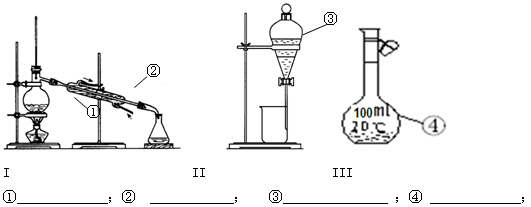


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��6.02��1023�ǰ����ӵ������Ľ���ֵ | B�������ӵ³������������ʵ�����1mol |
| C�������Ħ��������98�� | D��1mol 12Cԭ�ӵ�����Ϊ12g |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com