
��֪��һ�������£�R�DCl+2Na+Cl�DR���R�DR��+2NaCl
��ȩ�ʹ����Է���������Ӧ��������ʱ����������״����ǣ�
RCHO���ף�R�DCH2OH��֮��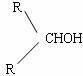 ����
����
����ij�ȴ���A������Է�������Ϊ78.5��B��������Ӧ���ɵ�һ�ȴ��������֡��йص�ת����ϵ����ͼ��ʾ�����ֲ��P����������ȥ����
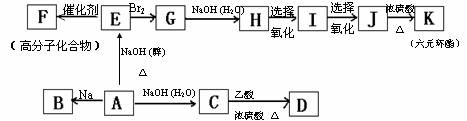
��ش��������⣺
��1������ʽ��A ����Ӧ���ͣ�A��C ��E��F ��
��2���ṹ��ʽ��B ��K ��
��3����Ӧ����ʽ��A��E ��
G��H ��
��4��D��ͬ���칹����������������֣�������2���DCH3���ŵ������֡���д���������е���һ�ֽṹ��ʽΪ ��
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com