
| m |
| M |
| N |
| N A |
| V |
| Vm |
| m |
| M |
| N |
| N A |
| V |
| Vm |
| m |
| 28 |
| m |
| 44 |
| m |
| 28 |
| m |
| 44 |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�꽭��ʡ����9�³���⻯ѧ�Ծ� ���ͣ������
��14�֣��±���Ԫ�����ڱ���һ���֣���Ա��еĢ١�����Ԫ�أ���Ԫ�ط��Ż�ѧʽ��ջش��������⣺
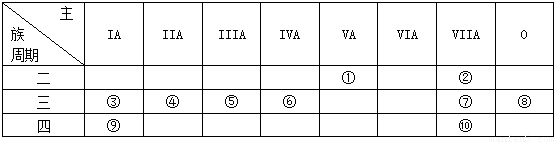
��1���ڢۡ���Ԫ���У�ԭ�Ӱ뾶��С���� �� �������ӵ�ԭ�ӽṹʾ��ͼΪ
��2������Ԫ�ص�����������Ӧ��ˮ������������ǿ���� �� ������
ǿ������ �������Ե����������� ��
��3����Ҫ��д�������������ʵĵ���ʽ��
�ں���Ԫ���γɵĻ���� ���۵Ĺ������ ��
��4���ڢ����ĵ����У���ѧ���ʽϻ��õ��� �������ӷ���ʽ֤������
ʵ��д����Ӧ�����ӷ���ʽ���� ��
(5)�õ���ʽ��ʾ����������Ӧ�õ��Ļ�������γɹ��� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010�������ʡ������ѧ�����п��ԣ���ѧ�� ���ͣ������
��14�֣��±���Ԫ�����ڱ���һ���֣���Ա��еĢ١�����Ԫ�أ���Ԫ�ط��Ż�ѧʽ��ջش��������⣺
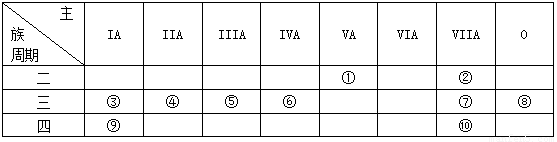
��1���ڢۡ���Ԫ���У�ԭ�Ӱ뾶��С���� �� �������ӵ�ԭ�ӽṹʾ��ͼΪ
��2������Ԫ�ص�����������Ӧ��ˮ������������ǿ���� �� ������
ǿ������ �������Ե����������� ��
��3����Ҫ��д�������������ʵĵ���ʽ��
�ں���Ԫ���γɵĻ���� ���۵Ĺ������ ��
��4���ڢ����ĵ����У���ѧ���ʽϻ��õ��� �������ӷ���ʽ֤������
ʵ��д����Ӧ�����ӷ���ʽ���� ��
(5)�õ���ʽ��ʾ����������Ӧ�õ��Ļ�������γɹ��� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010���½�ũ��ʦ����ѧ��һ�ڶ�ѧ�ڵڶ��ο��Ի�ѧ���� ���ͣ������
��14�֣��±���Ԫ�����ڱ���һ���֣���Ա��еĢ١�����Ԫ�أ���Ԫ�ط��Ż�ѧʽ��ջش��������⣺
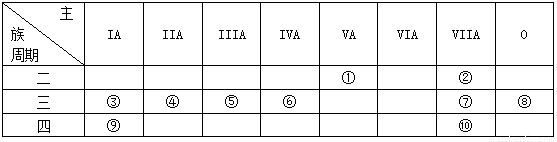
��1���ڢۡ���Ԫ���У�ԭ�Ӱ뾶��С���� �� �������ӵ�ԭ�ӽṹʾ��ͼΪ
��2������Ԫ�ص�����������Ӧ��ˮ������������ǿ���� �� ������
ǿ������ �������Ե����������� ��
��3����Ҫ��д�������������ʵĵ���ʽ��
�ں���Ԫ���γɵĻ���� ���۵Ĺ������ ��
��4���ڢ����ĵ����У���ѧ���ʽϻ��õ��� �������ӷ���ʽ֤������
ʵ��д����Ӧ�����ӷ���ʽ���� ��
(5)�õ���ʽ��ʾ����������Ӧ�õ��Ļ�������γɹ��� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij��ѧ��ȤС���ͬѧ������ͼ��ʾʵ��װ�ý���ʵ�飨ͼ��a��b��c��ʾֹˮ�У���

�밴Ҫ����գ�
��1������Bװ�ÿ���ȡ��������____________��д�����ּ��ɣ���
��2��A��C��E�������װ�ÿ�������ȡCl2��������ص�����ʵ�飮
�����ڱ��м�������ˮ�������Ƶ���ˮ����������ˮ��Ϊ���ݣ����Т�����ʵ
�飬ʵ����������������£�
| ʵ����� | ʵ����� | ���� | ���� |
| �� | ����ˮ����Ʒ����Һ | ��Һ��ɫ | ������ˮ��Ӧ�IJ�����Ư���� |
| �� | ��ˮ�м���̼�����Ʒ�ĩ | ����ɫ���ݲ��� | ������ˮ��Ӧ�IJ���������� |
��������ʵ���Ľ����Ƿ������������������˵������________________
________________________________.
������������װ�����һ����ʵ����֤Cl����Br���Ļ�ԭ��ǿ�����ֱ�ָ���ס��ҡ�����ʢ�ŵ��Լ���ʵ�������ۣ�____________
��3��B��D��Eװ����������B��ʢװŨ�����ͭƬ�������п����ϰ��ϣ������Ƶò�����NO2�й�ʵ�飮
��B�з�����Ӧ�Ļ�ѧ����ʽΪ______________________________________
������Dװ����֤NO2��ˮ�ķ�Ӧ�����������Ϊ���ȹر�ֹˮ��________���ٴ�ֹˮ��________��ʹ�ձ��е�ˮ�����Թܶ��IJ�����________________��
���Թܶ��е�NO2��ˮ��ַ�Ӧ�������Թ��ڻ���ͨ��һ������������ֱ���Թ�ȫ������ˮ����������Һ�����ʵ����ʵ���Ũ����________�����尴��״�����㣩��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����С�12�� ��ij��ѧ��ȤС���ͬѧ������ͼ��ʾʵ��װ�ý���ʵ�飨ͼ��a��b��c��ʾֹˮ�У���
��ij��ѧ��ȤС���ͬѧ������ͼ��ʾʵ��װ�ý���ʵ�飨ͼ��a��b��c��ʾֹˮ�У���

�밴Ҫ����գ�
��1������Bװ�ÿ���ȡ��������______________________��д�����ּ��ɣ���
��2��A��C��E�������װ�ÿ�������ȡCl2��������ص�����ʵ�飮
���ڱ��м�������ˮ�������Ƶ���ˮ����������ˮ��Ϊ���ݣ����Т�����ʵ
�飬ʵ����������������£�
| ʵ����� | ʵ����� | ���� | ���� |
| �� | ����ˮ����Ʒ����Һ | ��Һ��ɫ | ������ˮ��Ӧ�IJ�����Ư���� |
| �� | ��ˮ�м���̼�����Ʒ�ĩ | ����ɫ���ݲ��� | ������ˮ��Ӧ�IJ���������� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com