
ij����ѧϰС���ͬѧ�������ͼ��װ�ã�����֤SO2�������ԡ���ԭ�Ժ�Ư���ԡ�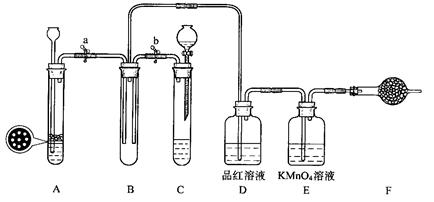
�ش��������⣺
33.�������װ��C�������ԵIJ����ǣ��رջ���b��Ȼ��________________________��
������________________��˵��װ��C���������á�
34.��Na2SO3�����������Һ��ȡSO2���壬Ӧѡ��__________��ѡ��A����C���������巢��װ�ã���ѡ����һװ�õ������� ��
35.С���ͬѧ��A��Cװ���е���һ����FeS�����ϡ������ȡH2S���壬��Ӧ�Ļ�ѧ����ʽΪ_________________ ��
36.SO2����ͨ��Dװ��ʱ������____________________ ��ͨ��Eװ��ʱ������
________________ ��SO2��H2S��Bװ���з�Ӧ��������______________________��
37.F��ʢ�м�ʯ�ң���������______________________��
33.���Թ��м�ˮ��1�֣�;��ˮ�Ӳ���ȥ��1�֣�
34.C��1�֣���Na2SO3������ˮ,SO2Ҳ������ˮ,�����������շ��������ܵ�װ��.��1�֣����1�㼴�ɣ�
35. FeS+H2SO4(ϡ)�� Fe SO4+H2S����1�֣���
36.��ɫ��ȥ��1�֣�;��ɫ��ȥ��1�֣�; ƿ���е���ɫ��ĩ����ɫ��СҺ�Σ�1�֣����1�㼴�ɣ�
37.����β����1�֣�
�������������33.���װ��C�������ԣ��رջ���b��Ȼ�����Թ��м�ˮ����ˮ�Ӳ���ȥ˵��װ��C���������á�
34.��ΪNa2SO3�ǹ����ĩ�����շ���������ʢ�Ų��ˣ���������Һ��ȡSO2���壬Ӧѡ�÷�Һ©���ͷ�Ӧ�������巢��װ�á�
35.��FeS�����ϡ������ȡH2S����ΪFeS+H2SO4(ϡ)�� Fe SO4+H2S����
36.SO2������Ư�����ã�ͨ��Dװ��ʱʹƷ����ɫ��SO2�����л�ԭ�ԣ�ͨ��Eװ��ʱʹ���Ը��������Һ��ɫ��SO2��H2S��Bװ���з�Ӧ��SO2+2H2S��3S+2H2O��ƿ���е���ɫ��ĩ����ɫ��СҺ�Ρ�
37. SO2��H2S�����������壬F��ʢ�м�ʯ�ң��������Ƕ����SO2��H2S��
���㣺SO2��H2S����ķ���װ�ü�����ʵ����������ۡ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ijͬѧ̽��SO2�й����ʡ�
��1����SO2ͨ��BaCl2��Һ�У���������Һ�ֳ�2�ݣ��ڵ�һ���м���NaOH��Һ���ڵڶ����е���FeCl3��Һ�����ݶ��а�ɫ������BaCl2��Һ��ͨ��SO2���������Ϊ ���ڵڶ�����Һ�е���FeCl3��Һʱ��SO2���� �ԣ���Ӧ�����ӷ���ʽΪ �����ɳ����Ļ�ѧʽΪ ��
��2���������������SO2������� ��
| A��KMnO4(aq) | B��������ˮ | C��ʯ��ˮ | D������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
�����14�֣�
���ж��ֺ����ᣬ�����ᣨH2SO3�������ᣨH2SO4���������ᣨH2SO4��SO3����������ᣨH2S2O3���ȵȣ�����������Ϊ��Ҫ���ڹ�ҵ���й㷺��Ӧ�á���ʵ���ң�Ũ�����dz��õĸ������
������м��㣺
(1)�����ᣨH2SO4��SO3������ˮ�����е�SO3��ת��Ϊ���ᡣ����445g����������ˮ���4.00L���ᣬ����������ʵ���Ũ��Ϊ________mol/��
(2)����Ũ������ˮ�����ɵ�H2SO4��H2O���㣬250g��������Ϊ98%�����������ն���gˮ��
(3)�������ǹ�ҵ�����������Ҫԭ�ϡ��������������յĻ�ѧ��Ӧ���£�
3FeS2��8O2��Fe3O4��6SO2 4FeS2��11 O2��2Fe2O3��8SO2
��48mol FeS2��ȫ��Ӧ��������2934.4L����״���������㷴Ӧ������Fe3O4��Fe2O3���ʵ���֮�ȡ�
(4)��������ȡ���ᣬ���ܳ��������Դ���ܱ�����������һ�ֺ��з�չǰ;���Ʊ�����ķ�����
�����������Ϊ0.84�Ļ�����壨H2S��H2O��N2���ڿ�������ȫȼ�գ�����������77%���������������SO2���������ˮ�����壩������֪������ɣ�N2�������0.79��O2�������0.21��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��ʵ�����п�����ͼ��ʾװ����ȡ����ء��������ƺ�̽����ˮ�����ʡ�
ͼ�У� ��Ϊ��������װ�ã��ڵ��Թ���ʢ��15mL 30�� KOH ��Һ����������ˮԡ�У� �۵��Թ���ʢ��15mL 8 % NaOH ��Һ�������ڱ�ˮԡ�У� �ܵ��Թ��������ɫʯ����Һ�� ��Ϊβ������װ�á�
����д���пհף�
��1����ȡ����ʱ������ƿ�����һ�����Ķ������̣�ͨ��______����д�������ƣ�����ƿ�м���������Ũ���ᡣʵ��ʱΪ�˳�ȥ�����е��Ȼ������壬���ڢ����֮�䰲װʢ��_________����д���б����ĸ���ľ���װ�á�
| A����ʯ�� | B������ʳ��ˮ | C��Ũ���� | D������̼��������Һ |

| ʵ������ | ԭ�� |
| ��Һ�������ɫ��Ϊ ɫ | ������ˮ��Ӧ���ɵ�H+ʹʯ���ɫ |
| �����Һ��Ϊ��ɫ | ______________________________________ |
| Ȼ����Һ����ɫ��Ϊ ɫ | _________________________________________ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��16�֣�ij�о���ѧϰС��Ϊ�о�������Ũ���ᷴӦ����������ijɷݲ��ⶨ������ĺ���������������ʵ�飺��ʵ���Ʋ⡿������������Ũ���ᷴӦ������SO2��H2�������塣
��1����С���������Ʋ�������ǣ� ��
��ʵ������a����ѡ���ҩƷ�����ۡ�Ũ���ᡢ����ͭ��ĩ��0.2 mol/L��H2C2O4����Һ��0.1 mol/L������KMnO4����Һ�����ָʾ����
b��ʵ��װ����Ƽ���װ(���ȼ��г�װ�þ�����ȥ)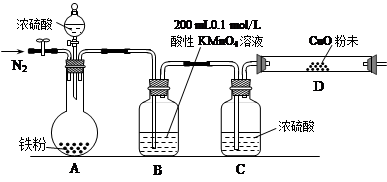
��ʵ����̼����ݴ�����
��2��B�з�����Ӧ�����ӷ���ʽΪ�� ��
��3��ʵ��ǰ��ͨ��һ��ʱ���N2����Ŀ���� ��
��4��B��C��D����ʢ�Լ�����������֤ʵ���ɵ�������ͬʱ����SO2��H2��������
��
��5��A�з�Ӧ��������ͨN2ʹA�����ɵ�����ȫ���ϳ�����B��D�з�Ӧ��ȫ���Ⱥ�����ȡ��B�з�Ӧ�����Һ����ƿ�У�ÿ��ȡ��25 mL����H2C2O4����Һ���вⶨ��
��H2C2O4����Һ������KMnO4��Һ��Ӧ�����ӷ���ʽ���£��뽫�÷���ʽ��ɲ���ƽ��
( )H2C2O4+( )MnO4- +( )H+ ��( )Mn2+ +( ) H2O+( ) ( )
�ڷ�Ӧ��ȫ�������� ��
���ظ��ⶨ���Σ�ƽ��ÿ�κ���H2C2O4����Һ15.63 mL��������Ũ���ᷴӦ������SO2����
�����ʵ���Ϊ ����������ʵ��ǰ��װ��D����������0.8 g���������������SO2���������Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��16�֣��������볱ʪ����ʯ�ҷ�Ӧ��ȡ����Ư�ۣ�Ҳ����Ũ��Һ��������ʯ��ʯ��Ӧ���ɽ�Ũ��HClO��Һ��
��.���о���ѧϰС����������������Ư�ۣ���ͼ��ʾ����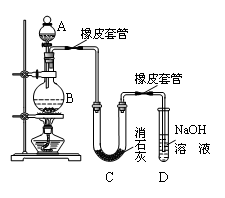
��1��A������������ ����ʢ�Լ��� ��
��2����ʵ��������Ca(ClO)2����̫�͡����������������Ϸ�����Ҫԭ������U���д�����������Ӧ��
���¶Ƚϸ�ʱ��������ʯ�ҷ�Ӧ������Ca(ClO3)2��Ϊ����˸���Ӧ�ķ������ɲ�ȡ�Ĵ�ʩ�� ���˸���Ӧ�Ļ�ѧ����ʽΪ�� ��
��д����һ������Ӧ�Ļ�ѧ����ʽ ��Ϊ����˸���Ӧ����������BC֮��Ӷ�һ��װ�ã����ڴ�����ķ����л�����װ�ã����������õ��Լ���
��. ���о���ѧϰС���о�������ˮ��ʯ��ʯ�ķ�Ӧ��
�����Թ��м�������Ŀ�״̼��ƣ��ټ���Լ20mL������ˮ����ͼ��ʾ������ַ�Ӧ�����������ݲ�������Һdz����ɫ��ȥ��
�ڹ��ˣ�����Һ������ɫ�����ϣ����������ˮ��Ư���Ը�ǿ��
��Ϊ��ȷ����Ӧ�������Һ��Ϊ���ݣ��ֱ��������ʵ�飺
��һ����ʯ��ˮ��ϣ���������������ɫ������
�ڶ�����ϡ�����ϣ���������������ɫ���壻
�������ݼ��ȣ�������Һ��������д�����ɫ��������� ����⣬����ʵ���в�������ɫ�����ΪCO2����ش�
��3����Ӧ�����õ���ҺƯ������ǿ��ԭ���� ��
��4����������ʵ�����֪���ڵ���Һ�е����ʳ�CaCl2��HClO�⣬������ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��ǹ�ҵ���Ʊ�Na2S2O3�ķ���֮һ����Ӧԭ��Ϊ��2Na2S+Na2CO3+4SO2=3Na2S2O3+CO2���÷�Ӧ��H��0����ij�о�С����ʵ��������Ʊ�Na2S2O3��5H2O�������¡�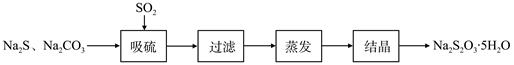
��1������װ����ͼ��ʾ��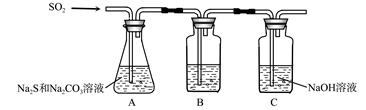
��װ��B�������Ǽ���װ��A��SO2������Ч�ʣ�B���Լ��� ������SO2����Ч�ʵ͵�ʵ��������B����Һ ��
��Ϊ��ʹSO2������������ȫ���ڲ��ı�A����ҺŨ�ȡ�����������£����˼�ʱ���跴Ӧ���⣬���ɲ�ȡ�ĺ�����ʩ�� �� ����д��������
��2�����豾ʵ�����õ�Na2CO3������NaCl��NaOH�����ʵ�鷽�����м��顣������ʱCaCO3������Һ��pH��10��2��
��ѡ�Լ���������ϡ���ᡢAgNO3��Һ��CaCl2��Һ��Ca(NO3)2��Һ����̪��Һ������ˮ��pH�ơ��ձ����Թܡ��ι�
| ��� | ʵ����� | Ԥ������ | ���� |
| �� | ȡ������Ʒ���Թ��У�������������ˮ��������ܽ⣬___________________�� | _______________ | ��Ʒ��NaCl |
| �� | ��ȡ������Ʒ���ձ��У�������������ˮ����ֽ����ܽ⣬___________________�� | _______________ | ��Ʒ��NaOH |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ijУ��ѧʵ����ȤС��Ϊ����֤��ʵ�����Ʊ�Cl2�Ĺ�������ˮ������HCl�ӷ�������ͬʱ֤��Cl2��ijЩ���ʣ���ͬѧ�������ͼ��ʾ��ʵ��װ��(֧���õ�����̨ʡ��)���밴Ҫ��ش��������⡣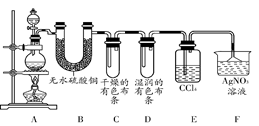
(1)���з����У����Ƶ�Cl2����ȷ����� ��
��MnO2��Ũ�����Ϲ��ȣ���MnO2��NaCl��Ũ�����Ϲ��ȣ���NaClO��Ũ�����ϣ���K2Cr2O7��Ũ�����ϣ���KClO3��Ũ�����Ϲ��ȣ���KMnO4��Ũ�����ϡ�
A���٢ڢ� B���ڢܢ�
C���٢ܢ� D��ȫ������
(2)д��ʵ������ȡCl2�����ӷ���ʽ ��
(3)��װ��B�������� ��
��װ��C��D���ֵIJ�ͬ����˵���������� ��
��װ��E�������� ��
(4)��ͬѧ��Ϊ��ͬѧ��ʵ����ȱ�ݣ�����ȷ������ͨ��AgNO3��Һ�е�����ֻ��һ�֡�Ϊ��ȷ��ʵ����۵Ŀɿ��ԣ�֤������ͨ��AgNO3��Һ�е�����ֻ��һ�֣���ͬѧ���Ӧ����װ�� �� ֮��(��װ����ĸ���)����һ��װ�ã�������װ��������Լ�����Ϊ (����ĸ���)��
A��ʪ��ĵ⻯�ص�����ֽ B��Ũ����
C��ʪ��ĺ�ɫ���� D������ʳ��ˮ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com