
���� ��1����������һ�����ʵ���Ũ����Һһ�㲽��ѡ����Ҫ����������������Һ���ѡ����ʹ������ƿ��
��2������m=CVM������Ҫ���ʵ�������
��3������ƿ���л�����ʹ�ù�������Ҫ���µߵ�ҡ�ȣ�����ʹ��ǰӦ����Ƿ�©ˮ��
��4���������������ʵ����ʵ�������Һ�����Ӱ�죬����C=$\frac{n}{V}$������������
��� �⣺��1������һ�����ʵ���Ũ����Һһ�㲽�裺���㡢�������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ��װƿ�ȣ��õ���������������ƽ��ҩ�ס�����������Ͳ���ձ�������ƿ����ͷ�ιܣ�����80mL 1.4mol•L-1��NaNO3��Һ��Ҫʹ��100mL����ƿ���ò�����������500mL����ƿ��
�ʴ�Ϊ��B��
��2����NaNO3��������80mL 1.4mol•L-1��NaNO3��Һ��Ӧѡ��100mL����ƿ��ʵ������100mL��Һ����Ҫ���ʵ�����m=85g/mol��1.4mol/L��0.1L=11.9g��
�ʴ�Ϊ��11.9��
��3������ƿ���л�����ʹ�ù�������Ҫ���µߵ�ҡ�ȣ�����ʹ��ǰӦ����Ƿ�©ˮ��
�ʴ�Ϊ������Ƿ�©ˮ��
��4��A�����ձ����ܽ����ʣ�����ʱ��������������Һ���������ʲ�����ģ����ʵ����� ����ƫС����ҺŨ��ƫ�ͣ�
B��δ��ϴ���ձ��ڱڵ���Һת��������ƿ���������ʲ�����ģ����ʵ����� ����ƫС����ҺŨ��ƫ�ͣ�
C������ƿ���������ҺҺ��δ���̶��߱�ֹͣ��ˮ��������Һ���ƫС����ҺŨ��ƫ�ߣ�
D����Һ���о�һ�ԣ�����õ���Һ������ƿת�Ƶ�����ྻ���Լ�ƿ��ʱ������������������ҺŨ����Ӱ�죻
E�����ձ�����Һת�Ƶ�����ƿ֮ǰ������ƿ������������ˮ�������ʵ����ʵ�������Һ������������Ӱ�죬��ҺŨ�Ȳ���Ӱ�죻
F������ƿ��Һ�潫�ﵽ�̶���ʱ�����ӿ̶��ߺ�Һ�棬������Һ���ƫС����ҺŨ��ƫ�ߣ�
���ԣ�������һ�����ʵ���Ũ����Һ��ʵ���У����в�����������ҺŨ����Ӱ�����DE����ʹ������ҺŨ��ƫ�����CF����ʹ������ҺŨ��ƫС����AB��
�ʴ�Ϊ��DE��CF��AB��
���� ���⿼����һ�����ʵ���Ũ����Һ�����ƣ���ȷ����ԭ�������������ǽ���ؼ���ע���������ķ����ͼ��ɣ�


 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
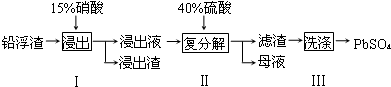
| ��ѧʽ | CaSO4 | Ag2SO4 | PbSO4 |
| Ksp | 4.9��10-5 | 1.2��10-5 | 1.6��10-8 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
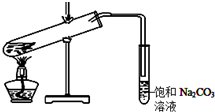 ijѧ���������������Ʊ�ʵ��װ����ͼ��ʾ��
ijѧ���������������Ʊ�ʵ��װ����ͼ��ʾ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
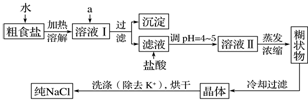
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | NaCl���壨KNO3���ܽ�����ȱ�����Һ�����½ᾧ | |
| B�� | CO��ˮ������ͨ��Ũ�����ϴ��ƿ | |
| C�� | CaO���壨CaCO3���������� | |
| D�� | KCl���壨MnO2���ܽ⡢���ˡ����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | H2O��g��=H2��g��+$\frac{1}{2}$ O2��g����H=+242 kJ/mol | B�� | 2H2��g��+O2��g��=2H2O ��l����H=-484 kJ/mol | ||
| C�� | H2��g��+$\frac{1}{2}$ O2��g��=H2O ��g����H=-242 kJ | D�� | 2H2��g��+O2��g��=2H2O ��g����H=+484 kJ/mol |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ʵ���ҽ�����ͭ��Һֱ�Ӽ�����������ȡCuSO4•5H2O | |
| B�� | ��ʪ���pH��ֽ������Һ��pH | |
| C�� | ������Ũ����Һմ��Ƥ���ϣ�����������ϡ�����кͣ����ô���ˮ��ϴ����Ϳ��2%��5%��������Һ | |
| D�� | �����Ȼ�̼��ȡ��ˮ�е��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 60g C2H4O2�������õ��Ӷ���ĿΪ7NA | |
| B�� | 1L 0.1mol•L-1������Һ��H+��Ϊ0.1NA | |
| C�� | 1mol����������������Ϊ9NA | |
| D�� | ��״���£�22.4L�Ҵ��ķ�����ΪNA |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com