
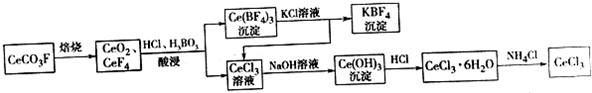
���� ���÷�̼�����Ҫ�ɷ�CeCO3F����ȡCeCl3��һ�ֹ�������Ϊ��CeCO3F���յ�CeO2��CeF4���������������������˵ó���Ce��BF��4��CeCl3��Һ��Ce��BF��4���Ȼ�����Һ��Ӧ�õ�CeCl3��KBF��CeCl3��ǰ����Һ��CeCl3�ĺϲ��ټ��������ƣ���Ce��OH��3������Ce��OH��3���������ᷴӦ�ɵ�CeCl3•6H2O������CeCl3•6H2O��NH4Cl�Ĺ�������ɵõ���ˮCeCl3��
��1�����ݻ��ϼ۴�����Ϊ0�жϣ�
��2��������������H2O2�ܻ�ԭCeO2����Ce3+������Ԫ���غ�͵���غ���д���ӷ���ʽ��
��3����Ce��BF4��3�м���KCl��Һ����CeCl3��KBF����ֹ����Ce��BF4��3������
��4����Һ�е�C��Ce3+������1��10-5mol•L-1������ΪCe3+������ȫ������KSP[Ce��OH��3]=C��Ce3+��•C3��OH-�� �ɼ������Һ�����������ӵ�Ũ�ȣ�����ȷ��PHֵ��
��5��NH4Cl�������ȷֽ����HCl����������CeCl3ˮ�⣬�ݴ˴��⣻
��6����0.1000mol•l-1��NH4��2Fe��SO4��2����Һ���25.00ml�������������ӵ����ʵ���Ϊ2.5��10-3mol�����ݷ�ӦFe2++Ce4+�TCe3++Fe3+������CeԪ���غ��֪���ɼ����CeCl3������������ȷ����Ʒ��CeCl3������������
����ʹ�þ��õģ�NH4��2Fe��SO4��2����Һ���еζ��������������ӱ��������������������Ի����ı�Һ�����Ϊƫ�ݴ˷�����
��� �⣺��1�����ݻ��ϼ۴�����Ϊ0��֪��CeCO3F�У�CeԪ�صĻ��ϼ�Ϊ+3�ۣ�
�ʴ�Ϊ��+3��
��2��ϡ���ᡢH2O2��CeO2��Ӧ�����ӷ���ʽΪ��H2O2+2CeO2+6H+=2Ce3++4H2O+O2����
�ʴ�Ϊ��H2O2+2CeO2+6H+=2Ce3++4H2O+O2����
��3����Ce��BF4��3�м���KCl��Һ����CeCl3��KBF����������Ŀ���DZ�����������Ce��BF4��3��������ʽ��ʧ���ȥBF4-�����CeCl3�IJ��ʣ�
�ʴ�Ϊ��������������Ce��BF4��3��������ʽ��ʧ���ȥBF4-�����CeCl3�IJ��ʣ�
��4����Һ�е�C��Ce3+������1��10-5mol•L-1������ΪCe3+������ȫ������KSP[Ce��OH��3]=C��Ce3+��•C3��OH-��=1��10-20��֪��C��OH-��=$\root{3}{\frac{1��1{0}^{-20}}{1��1{0}^{-5}}}$mol•L-1=1��10-5mol•L-1����ʱ��Һ��PHΪ9��
�ʴ�Ϊ��9��
��5������NH4Cl�������ȷֽ����HCl������CeCl3ˮ�⣬���Լ���CeCl3•6H2O��NH4Cl�Ĺ�������ɵõ���ˮCeCl3��
�ʴ�Ϊ��NH4Cl�������ȷֽ����HCl������CeCl3ˮ�⣻
��6����0.1000mol•l-1��NH4��2Fe��SO4��2����Һ���25.00ml�������������ӵ����ʵ���Ϊ2.5��10-3mol�����ݷ�ӦFe2++Ce4+�TCe3++Fe3+������CeԪ���غ��֪��CeCl3������Ϊ2.5��10-3mol��246.5g/mol=0.6163g��������Ʒ��CeCl3����������Ϊ$\frac{0.6163g}{0.7500g}$��100%=82.2%��
�ʴ�Ϊ��82.2%��
����ʹ�þ��õģ�NH4��2Fe��SO4��2����Һ���еζ��������������ӱ��������������������Ի����ı�Һ�����Ϊƫ�����ø�CeCl3��Ʒ����������ƫ��
�ʴ�Ϊ��ƫ��
���� ��������ȡCeCl3��һ�ֹ�������Ϊ���壬�����˻��ϼۡ����ӷ�Ӧ�������ܽ�ƽ��ļ��㡢ʵ�������������ѧ�����֪ʶ�����ط������ƶϼ�ʵ���������ۺϿ��飬��Ŀ�Ѷ��еȣ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
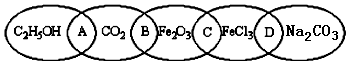
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
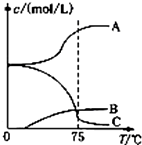
 NaH2PO4��Na2HPO4�����ںϳɻ���ԭ�����������ƣ�Na5P3O10����
NaH2PO4��Na2HPO4�����ںϳɻ���ԭ�����������ƣ�Na5P3O10����| �ζ����� | ������ҺA�������/mL�� | 0.1000mol•L-1NaOH��Һ����� | |
| �ζ�ǰ������/mL�� | �ζ��������/mL�� | ||
| 1 | 25.00 | 1.02 | 21.03 |
| 2 | 25.00 | 2.00 | 21.99 |
| 3 | 25.00 | 0.20 | 20.20 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ʽ�ζ���ֻ��ˮϴ����δ�ô���Һ��ϴ | |
| B�� | ��ƿ�в���������ˮ | |
| C�� | ��ʽ�ζ��ܵζ�ǰ���촦�����ݣ��ζ���������ʧ | |
| D�� | �ζ�ǰ���ӿ̶ȶ������ζ����ӿ̶ȶ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 �����ƣ�NaClO3�������ι�ҵ����Ҫ��Ʒ֮һ��
�����ƣ�NaClO3�������ι�ҵ����Ҫ��Ʒ֮һ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ij��ѧѧϰС���ͬѧ ����������ԭ��Ӧ��MnO4-+5Fe2++8H+�TMn2++5Fe3++4H2O���ɲ��õζ��ķ����ⶨFeSO4������������ʵ�鲽�����£�
ij��ѧѧϰС���ͬѧ ����������ԭ��Ӧ��MnO4-+5Fe2++8H+�TMn2++5Fe3++4H2O���ɲ��õζ��ķ����ⶨFeSO4������������ʵ�鲽�����£��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �ñ�FeCl3��Һ�ζ�KI��Һ��ѡ��KSCN��Һ | |
| B�� | ��I2��Һ�ζ�Na2SO3��Һ��������ָʾ�� | |
| C�� | ��AgNO3��Һ�ζ�NaCl��Һ��Na2CrO4��ָʾ�� | |
| D�� | ��H2O2��Һ�ζ�KI��Һ��������ָʾ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com