
��8�֣���ͼ����Уʵ���һ�ѧ�Լ�Ũ�����ǩ�ϵIJ������ݡ�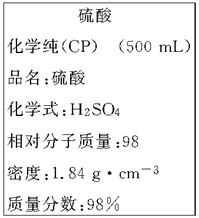
����Ҫ480 mL 1 mol��L-1��ϡ���ᡣ�ø�Ũ���������ˮ���ƣ��ɹ�ѡ�õ������У�
�ٽ�ͷ�ιܣ��ڲ����������ձ�������Ͳ��
��ش��������⣺
��1������ϡ����ʱ����ȱ�ٵ�������_________________(д��������)��
��2�������㣬����Ũ��������ԼΪ________mL��������Ũ������������ˮ��ϣ�������Һ�����ʵ���������____49%(�>�� ����<�� ����)��
��3���������Ƶ�ϡ������вⶨ��������Ũ�ȴ���1 mol��L��1�����ƹ��������и�������������������ԭ����________��
������Ͳ��ȡŨ����ʱ�����ӿ̶���
������ƿ������ˮϴ�Ӻ�δ������
������Ͳ��ȡŨ�����������ˮ����Ͳϴ�Ӹɾ���ϴ��Һת�Ƶ��ձ���
��ת����Һʱ��������������Һ����
�ݶ���ʱ����������ƿ�̶���
���ݺ�����ƿ����ҡ�ȣ����ź���Һ����ڿ̶��ߣ��ټ�����ˮ���̶���
��1��500 mL������ƿ ;��2��27.2 ; > ;��3���٢ۢ�
�����������������1������˳���ǣ��������ȡ��ϡ�͡���ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȡ�װƿ��ǩ��һ������Ͳ���õ���ͷ�ιܣ���ȡ�����ձ���ϡ�ͣ���ȴ��ת�Ƶ�100mL����ƿ�У����ò�����������ת����ϣ�����������ˮϴ���ձ���������2��3�β���ϴ��Һȫ��ת�Ƶ�����ƿ�У��ټ���������ˮ������ˮ��Һ�����̶���1��2cmʱ�����ý�ͷ�ιܵμӣ�ʹ��Һ�İ�Һ�����͵��������ƽ������ƿ�����������µߵ�ҡ�ȣ�������Ҫ������Ϊ����Ͳ����ͷ�ιܡ��ձ�����������100mL����ƿ���ʴ�Ϊ��100 mL������ƿ���������� ��2����ͼ��ʾŨ�����Ũ��Ϊ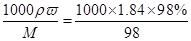 =18.4mol/L.ϡ��ǰ�����ʵ����ʵ����������仯����18.4mol/LxV=480mlx1mol/L�����V=27.2ml��������Ũ������������ˮ��ϣ��������ʵ������������仯����������Ϊ
=18.4mol/L.ϡ��ǰ�����ʵ����ʵ����������仯����18.4mol/LxV=480mlx1mol/L�����V=27.2ml��������Ũ������������ˮ��ϣ��������ʵ������������仯����������Ϊ ������������ܶȴ���ˮ���ܶȣ����������������������ˮ����������������������49%����3������c��n/V��֪��������Ͳ��ȡŨ����ʱ�����ӿ̶���ȡŨ���ᣬ���������ӣ�Ũ��ƫ�ߣ�������ƿ������ˮϴ�Ӻ�δ���������������ˮ����Ӱ��ʵ��������������ˮϴ����Ͳ����ת�Ƶ��ձ��У���ʹ���ʵ����ʵ���ƫ��Ũ��ƫ�ߣ���ת����Һʱ��������������Һ��������ƿ���棬�����ʼ��٣�Ũ��ƫС���ݶ���ʱ����������ƿ�̶��߽��ж��ݣ�������ƿ����Һ������٣�Ũ��ƫ�ߣ����ݺ�����ƿ����ҡ�Ⱥ���Һ����ڿ̶��ߣ��㲹�伸��ˮ���̶ȴ���������ƿ����Һ������ӣ�Ũ��ƫС���ʴ�Ϊ�٢ۢ�.
������������ܶȴ���ˮ���ܶȣ����������������������ˮ����������������������49%����3������c��n/V��֪��������Ͳ��ȡŨ����ʱ�����ӿ̶���ȡŨ���ᣬ���������ӣ�Ũ��ƫ�ߣ�������ƿ������ˮϴ�Ӻ�δ���������������ˮ����Ӱ��ʵ��������������ˮϴ����Ͳ����ת�Ƶ��ձ��У���ʹ���ʵ����ʵ���ƫ��Ũ��ƫ�ߣ���ת����Һʱ��������������Һ��������ƿ���棬�����ʼ��٣�Ũ��ƫС���ݶ���ʱ����������ƿ�̶��߽��ж��ݣ�������ƿ����Һ������٣�Ũ��ƫ�ߣ����ݺ�����ƿ����ҡ�Ⱥ���Һ����ڿ̶��ߣ��㲹�伸��ˮ���̶ȴ���������ƿ����Һ������ӣ�Ũ��ƫС���ʴ�Ϊ�٢ۢ�.
���㣺һ�����ʵ���Ũ����Һ������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
�����������÷����У�ռ�ҹ���������������
| A������ | B�����շ� | C���ѷ� | D������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ʵ������Ҫ0.5 mol��L��1������Һ450 mL���ɹ�ѡ��������У�
�ٲ����� ����ƿ ���ձ� �ܽ�ͷ�ι� ����Ͳ ������ƿ ��������ƽ ���Һ©��
ʵ����������������Ϊ98%���ܶ�Ϊ1.84 g��cm��3��ŨH2SO4�ṩ������������Һ����������ش��������⣺
��1�����������У�һֱ�ò������� ������ţ���
��2�����в����У�����ƿ���߱��Ĺ�����______________������ţ���
| A������һ�����ȷŨ�ȵı���Һ |
| B������������Һ |
| C�����������ܽ�������� |
| D����Ϊ��Ӧ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��10�֣���1��ʵ����Ҫ����100 mL 2 mol/L NaCl��Һ����ش��������⣺
��1�����ƹ�������Ҫʹ�õ���Ҫ�������������ձ�������������ͷ�ιܡ���Ͳ��___________��
��2����������ƽ��ȡ�Ȼ��ƹ��壬������Ϊ__________ g��
��3������Ҫ�����������ȷ˳����___________������ţ���
�ٳ�ȡһ���������Ȼ��ƣ������ձ��У�����������ˮ�ܽ⣻
�ڼ�ˮ��Һ��������ƿ���̶�����1~2����ʱ�����ý�ͷ�ιܵμ�����ˮ����Һ����̶������У�
�۽���Һת�Ƶ�����ƿ�У�
�ܸǺ�ƿ�����������µߵ���ҡ�ȣ�
������������ˮϴ���ձ��ڱںͲ�����2~3�Σ�ϴ��Һת�Ƶ�����ƿ�С�
��4�����ʵ�������ȱ�ٲ���ݣ������������Һ�����ʵ���Ũ��_______(�ƫ�ߡ���ƫ�͡�����Ӱ�족����ͬ)��������ʱ��������ƿ�̶� �ߣ������������Һ�����ʵ���Ũ��_________��
��2��ʵ���ҿ�����ͼװ���Ʊ���������ˮ��
���¶ȼ�ˮ������ ����ȫ���ϸ���ƺ��¶�
��Һ������������ƿ�����1/3��Ͷ���������Ƭ����ֹ_________��
����ͨ����ˮ���ټ��ȣ��������е�ˮ������ӦΪ ��
�ܸտ�ʼ�ռ��������Ӧ��ȥ������ʱ�� ���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
������480mL 0.2mol/L��NaCl��Һ���ش��������⣺
��1��Ӧ��������ƽȷ��ȡ����NaCl g��
��2��������ʵ��������裬��ȷ�IJ���˳���ǣ�
�ٳ�ȡ����������NaCl
�ڽ���Һת�Ƶ�����ƿ��
��������ˮϴ���ձ�2��3�Σ���ϴ��Һȫ����������ƿ�У�ҡ��
�ܸ��ý�ͷ�ιܣ�С�ĵ�������ˮ���̶�
����ϸ�ذ�����ˮע������ƿ�У�ֱ��Һ��ӽ��̶���1cm��2cm��
������ƿ�����������µߵ���ҡ��
�߽���ȡ��NaCl���壬����������ˮ�������ܽ����ȴ
��3����ʵ��������������������Ƶ���ҺŨ��ƫС�IJ�����
| A���ձ���NaCl��Һ��������ƿ��û��ϴ���ձ� |
| B�����ݺ�ҡ�ȣ�����Һ����ڿ̶��ߣ��ٵμ�����ˮ���̶��� |
| C��ʵ���õ�����ƿϴ����δ������溬������ˮ |
| D������ʱ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ijͬѧ�õ������壨CuSO4��5H2O ������0.040mol/L��CuSO4��Һ240mL���ش��������⣺
��1����������Ϊ��������ƽ��ҩ�ס��ձ�����Ͳ����ͷ�ιܣ�����Ҫ��Щ��������������ɸ�ʵ�飬��д���� ��
��2����д����ʵ��ļ�Ҫ��ʵ�鲽�裺
�ټ���ڳ������� g�� ��ת�Ƣ�ϴ�Ӳ�ת�Ƣ��ݢ�ҡ��
��3����ͼ�Ǹ�ͬѧת����Һ��ʾ��ͼ��ͼ�еĴ����� ��
��4����ͬѧ�ڶ��ݺӸǵ�תҡ�Ⱥ���Һ����ڿ̶��ߣ��ֵμ�����ˮ���̶ȡ���������ҺŨ�ȵ�Ӱ�� ���ƫ�ߡ�����ƫ�͡�����Ӱ�족����
��5��һƿ���ƺõ�ϡCuSO4��Һ����ʱ���������ǩ����Ϊ����������ʢ��CuSO4��Һ����д������SO42-ʵ����������� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��14�֣�ʵ�������ܶ�Ϊ1��19g/mL����������Ϊ36��5% Ũ��������250mL0��1mol/L��������Һ,��ղ���ش��������⣺
��1������250mL0��1mol/L��������Һ
| Ӧ��ȡ�������/mL | Ӧѡ������ƿ�Ĺ��/mL | ������ƿ���Ҫ���������� |
| | | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��6�֣�ÿС��2�֣����о������ʣ�1�����ߣ�2��ʯī��3��������4������þ���壨5�������ᣨ6�����ʯ��7��ʯ��ˮ��8���Ҵ���9�����ڵ������
�����ܵ������ �����ڵ���ʵ���
�Ȳ��ǵ����Ҳ���Ƿǵ���ʵ��� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
�����й��������ʵ�Ӧ�ô������
| A���ƾ��к�ǿ�Ļ�ԭ�ԣ���������TiCl4��Ӧ��ȡ�� |
| B��ͨ�����ȷ�Ӧ�����������Ӹֹ� |
| C���������ƿ�����CO2�� H2O��Ӧ��������DZˮͧ������ |
| D��Al2O3���кܸߵ��۵㣬���������������ռ������ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com