
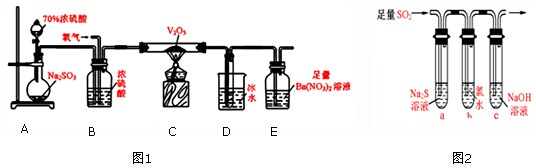
| mg |
| 80g/mol |
| m |
| 80 |
| ng |
| 233g/mol |
| n |
| 233 |
| ||||
|
| ||||
|


 �ܿ�����ĩ��̾�ϵ�д�
�ܿ�����ĩ��̾�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A����NaAlO2��Һ��ͨ�����CO2��Al��OH��3��AlO2-+CO2+2H2O=Al��OH��3��+HCO3- |
| B��Fe��OH��3��������Fe��OH��3+3H+=Fe3++3H2O |
| C��AlCl3��Һ�м��������Ũ��ˮ��Al3++4NH3?H2O��AlO2-+4NH4++2H2O |
| D��H2SO4��Ba��OH��2��Һ��Ӧ��Ba2++2OH-+2H++SO42-=BaSO4��+H2O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A����һ���¶��£�AgClˮ��Һ��Ag+��Cl-Ũ�ȵij˻���һ������ |
| B�������������뻯ѧ��Ӧ����Ӧǰ����������ɺ����ʶ����ֲ��� |
| C��Ӧ�ø�˹���ɣ��ɼ�Ӽ���ijЩ����ֱ�Ӳ����ķ�Ӧ�� |
| D�����ڷ��Ӽ�����Ĵ��ڣ���VA���⻯��ķе��С��ϵΪ��NH3��SbH3��AsH3��PH3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
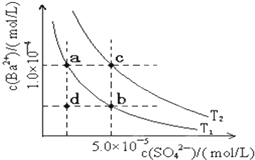 �±��Ǽ���������ʵĵ���ƽ�ⳣ�������ܵ���ʵ��ܶȻ�Ksp��25�棩��
�±��Ǽ���������ʵĵ���ƽ�ⳣ�������ܵ���ʵ��ܶȻ�Ksp��25�棩��| ����� | ƽ�ⷽ��ʽ | ƽ�ⳣ��K | Ksp |
| CH3COOH | CH3COOH?CH3COO-+H+ | 1.76��10-5 | |
| H2CO3 | H2CO3?H++HCO3- HCO3-?H++CO32- | K1=4.31��10-7 K2=5.61��10-11 | |
| C6H5OH | C6H5OH?C6H5O-+H+ | 1.1��10-10 | |
| H3PO4 | H3PO4?H++H2PO4- H2PO4-?H++HPO32- HPO42-?H++PO43- | K1=7.52��10-3 K2=6.23��10-8 K3=2.20��10-13 | |
| NH3?H2O | NH3?H2O?NH4++OH- | 1.76��10-5 | |
| BaSO4 | BaSO4?Ba2++SO42- | 1.07��10-10 | |
| BaCO3 | BaCO3?Ba2++CO32- | 2.58��10-9 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��BaCl2��Һ��ϡH2SO4 |
| B��NH4Cl��Һ��NaOH��Һ |
| C��K2CO3��Һ��ϡH2SO4 |
| D��KI��Һ��NaCl��Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
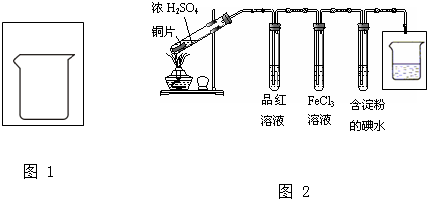
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
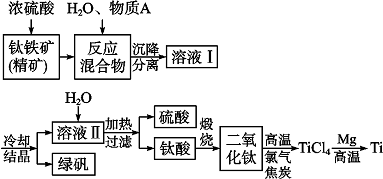
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��1 | B��3 | C��4 | D��6 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
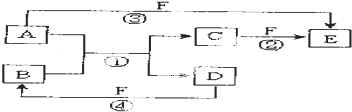
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com