
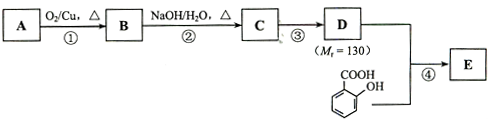
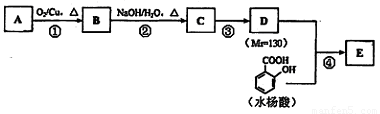
��֪��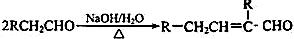 ��ˮ������EΪ�������ռ������������Ʒ�ɹ˪��E��һ�ֺϳ�·�����£�
��ˮ������EΪ�������ռ������������Ʒ�ɹ˪��E��һ�ֺϳ�·�����£�
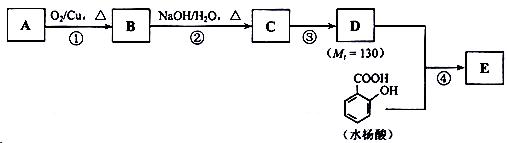
��ش��������⣺
��1��һԪ��A��������������ԼΪ21.6%����A�ķ���ʽΪ ���ṹ������ʾAֻ��һ������A������Ϊ ��
��2��B�������Ƶ�Cu(OH)2������Ӧ���÷�Ӧ�Ļ�ѧ����ʽΪ ��
��3��C�� �ֽṹ����һ��ȡ��������C�����������ţ���ʹ�õ��Ⱥ�˳��д�������Լ���
��
��4���ڢ۵ķ�Ӧ����Ϊ ��D���������ŵ�����Ϊ ��
��5��д��ͬʱ��������������ˮ��������ͬ���칹��Ľṹ��ʽ�� ��
a�������к���6��̼ԭ����һ������ b�����������������Ű���ˮ������еĹ�����
��6���ڢܲ��ķ�Ӧ����Ϊ ��д��E�Ľṹ��ʽ ��
��1��C4H1OO ��1-������������������
��2��CH3CH2CH2CHO+2Cu(OH)2+NaOH CH3CH2CH2COONa+Cu2O��+3H2O��
CH3CH2CH2COONa+Cu2O��+3H2O��
��3��2��������Һ��ϡ���ᡢ��ˮ�������������𰸣���
��4����ԭ��Ӧ����ӳɷ�Ӧ�����ǻ���
��5�� ��
��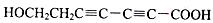 ��
��
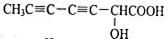 ��
��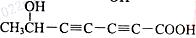 ��
��
��6��Ũ���ᡢ���ȣ�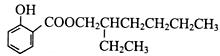
��������
��1����ΪһԪ�������ɺ�̼��������21.6%�������A����Է���������16/21.6%=74����A�ķ���ʽΪ��C4H1OO��ֻ��һ������Ϊ��CH3(CH2)3OH����������
��2���������ת����֪BΪ��ȩ��д��Ӧʱע�ⰴ�̲IJ�NaOH��CH3CH2CH2CHO+2Cu(OH)2+NaOH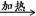 CH3CH2CH2COONa+Cu2O��+3H2O����
CH3CH2CH2COONa+Cu2O��+3H2O����
��3����������Ϣ��֪C����Ϊ�� ��
��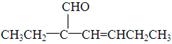 ��ͬʱ����ȩ����̼̼˫�����ȼ���ȩ������Ӧ������ȩ�����кͼ��ԣ��ټ���ˮ����̼̼˫����ע��˳����Ū������Ϊ��ˮҲ������ȩ������ɫ��
��ͬʱ����ȩ����̼̼˫�����ȼ���ȩ������Ӧ������ȩ�����кͼ��ԣ��ټ���ˮ����̼̼˫����ע��˳����Ū������Ϊ��ˮҲ������ȩ������ɫ��
��4������C���ֽṹ����������������ԭ��Ӧ���õ���Է�������Ϊ130�ľ�Ϊ�� ��
��
��5��ˮ����ķ���ʽΪ��C7H6O3,�����Ͷ�Ϊ5������Ŀ������������֪�����뺬���Ȼ����ǻ�����������̼̼����������ֲ���ʹ6��̼ԭ�ӹ��ߣ���д�ṹ��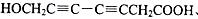 ��
�� ��
��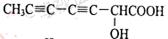 ��
��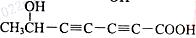 ����ͼ��֪��Ӧ����������Ӧ������ΪŨ���ᡢ���ȣ������ǻ���������ɵõ��������Ľṹ��
����ͼ��֪��Ӧ����������Ӧ������ΪŨ���ᡢ���ȣ������ǻ���������ɵõ��������Ľṹ��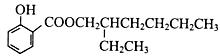
���������ɡ���1����Է��������ļ���ɲ�����һ��������ʽ������ɲ���ͨʽ������2����Ϣ����ʽ����д�ɲ����и���ҳ�̼�Ǽܷ����仯��λ�ã���3�������ŵļ���ע����������⣻��4��ͬ���칹�����д���Բ��ò����Ͷȷ���
�����㶨λ�����⿼���л���ѧ�ۺϣ��漰���㣺��Է��������ļ��㡢����ʽ��ȷ�����ṹ�ƶϡ���������Ϣ�����Ӧ�á�����ʽ����д�������ŵļ��顢��Ӧ�����жϡ������ŵ���д��ͬ���칹�����д����Ӧ�����жϡ�����ṹ��ʽ��д���ۺ��ԱȽ�ǿ������࣬�����ѶȲ��������֡�


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
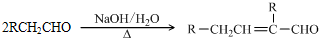
| �� |
| �� |
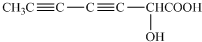
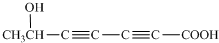
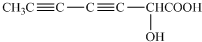
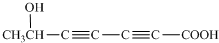
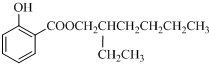
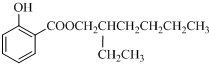
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪![]()
ˮ������EΪ�������ռ������������Ʒ�ɹ˪��E��һ�ֺϳ�·�����£�
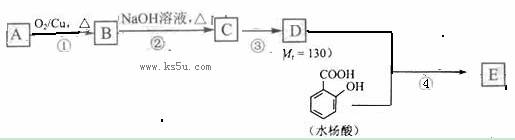
��ش��������⣺
��1�� һԪ��A��������������ԼΪ21.6%����A�ķ���ʽΪ�����ṹ������ʾAֻ��һ������A������Ϊ������������
��2�� B�������Ƶ�Cu(OH)2������Ӧ���÷�Ӧ�Ļ�ѧ����ʽΪ��������
��3�� C�С����ֽṹ����һ��ȡ��������C�����������ţ���ʹ�õ��Ⱥ�˳��д�������Լ�����������������
��4�� �ڢ۵ķ�Ӧ����Ϊ��������������D���������ŵ�����Ϊ������������
��5�� д��ͬʱ��������������ˮ��������ͬ���칹��Ľṹ��ʽ��������������
a�� �����к���6��̼ԭ����һ�����ϣ�
b�� ���������������Ű���ˮ������еĹ�����
��6���ڢܲ��ķ�Ӧ����Ϊ������������������д��E�Ľṹ��ʽ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013��ȫ����ͨ�ߵ�ѧУ����ͳһ���������ۺ��������Ի�ѧ�������������� ���ͣ������
��֪��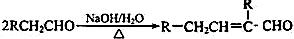 ��ˮ������EΪ�������ռ������������Ʒ�ɹ˪��E��һ�ֺϳ�·�����£�
��ˮ������EΪ�������ռ������������Ʒ�ɹ˪��E��һ�ֺϳ�·�����£�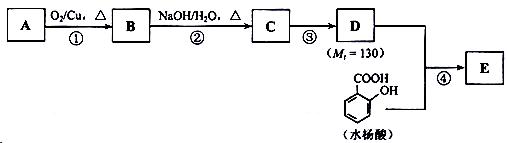
��ش��������⣺
��1��һԪ��A��������������ԼΪ21.6%����A�ķ���ʽΪ ���ṹ������ʾAֻ��һ������A������Ϊ ��
��2��B�������Ƶ�Cu(OH)2������Ӧ���÷�Ӧ�Ļ�ѧ����ʽΪ ��
��3��C�� �ֽṹ����һ��ȡ��������C�����������ţ���ʹ�õ��Ⱥ�˳��д�������Լ���
��
��4���ڢ۵ķ�Ӧ����Ϊ ��D���������ŵ�����Ϊ ��
��5��д��ͬʱ��������������ˮ��������ͬ���칹��Ľṹ��ʽ�� ��
a�������к���6��̼ԭ����һ������ b�����������������Ű���ˮ������еĹ�����
��6���ڢܲ��ķ�Ӧ����Ϊ ��д��E�Ľṹ��ʽ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014ѧ�����ʡ�����и�����ҵ��������飨�������ۻ�ѧ�Ծ��������棩 ���ͣ������
��֪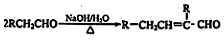 ˮ������EΪ���������ռ������������Ʒ�ɹ˪��E��һ�ֺϳ�·�����£�
ˮ������EΪ���������ռ������������Ʒ�ɹ˪��E��һ�ֺϳ�·�����£�
��ش��������⣺
��1��һԪ��A��������������ԼΪ21.6%����A�ķ���ʽΪ?????????????? ���ṹ������ʾAֻ��һ������A������Ϊ??????????? ��
��2��B�ܷ���������Ӧ���÷�Ӧ�Ļ�ѧ����ʽΪ?????????????????????????????? ��
��3��C��??????? �ֽṹ����һ��ȡ��������C�����������ţ���ʹ�õ��Ⱥ�˳��д�������Լ�??????????????????????????????? ��
��4���������ķ�Ӧ����Ϊ???????????????? ��D���������ŵ�����Ϊ??????????????? ��
��5��д��ͬʱ��������������ˮ�����ͬ���칹��Ľṹ��ʽ������дһ�֣� ????????? ��
a�������к���6��̼ԭ����һ�����ϣ�
b�����������������Ű���ˮ������еĹ�����
��6��д��E�Ľṹ��ʽ????????????????????????? ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com